Additionally, the region is expected to witness high growth rate during the forecast period due to the continuous efforts towards diabetes control by the government and healthcare organizations such as the IDF. These organizations provide required expertise and support diabetes awareness campaigns through a network of stakeholders and partners. Also, increasing health care funding in the region is anticipated to propel the market’s growth.
Moreover, rising geriatric population is also anticipated to propel the market growth over the forecast period. In 2018, nearly one fifth of the Europe’s population was over 65 years age which is further fueling the diabetes device market. According to the United Nation report on World Population Aging, older population in Europe is projected to constitute 35.0% of total population by 2050. Furthermore, leading manufacturers are focusing on technological innovations and advanced product development to gain remarkable share in the market.
Europe Diabetes Care Devices Market Report Highlights
- Germany diabetes care device market is expected to grow rapidly during the forecast period, owing to favorable reimbursement policies, local presence of key market players, and supportive government initiatives.
- Blood glucose monitoring (BGM) devices are expected to register significant growth over the forecast period, registering a CAGR of 6.5% over the forecast period.
- In 2023, Hospital pharmacies dominated the market and accounted for a share of 54.1%.
- Insulin pumps segment is expected to grow during the forecast period. The segment is primarily driven by its advantage such as need of fewer injections to deliver insulin.
The leading players in the Europe Diabetes Care Devices market include:
- Medtronic plc
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd.
- Bayer AG
- LifeScan IP Holdings, LLC
- B. Braun Melsungen AG
- Dexcom, Inc.
- Insulet Corporation
- Ypsomed Holding AG
- Companion Medical, Inc.
- Sanofi S.A.
- Valeritas Holdings, Inc.
- Novo Nordisk A/S
- Arkray, Inc.
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The leading players in the Europe Diabetes Care Devices market include:- Medtronic plc
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd.
- Bayer AG
- LifeScan IP Holdings, LLC
- B. Braun Melsungen AG
- Dexcom, Inc.
- Insulet Corporation
- Ypsomed Holding AG
- Companion Medical, Inc.
- Sanofi S.A.
- Valeritas Holdings, Inc.
- Novo Nordisk A/S
- Arkray, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 100 |
| Published | September 2024 |
| Forecast Period | 2023 - 2030 |
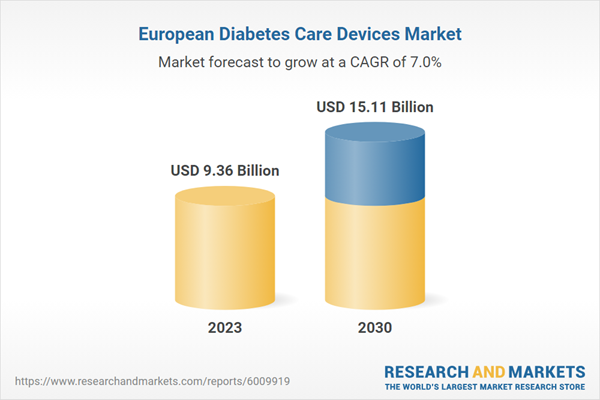
| Estimated Market Value ( USD | $ 9.36 Billion |
| Forecasted Market Value ( USD | $ 15.11 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 15 |