Key Takeaways
- Ruptured intracranial aneurysms are considered fatal in around 50% of cases, with recent data suggesting that about 25% of people die within the first 24 hours of the rupture and nearly 50% die within 3 months . The growing demand for effective treatment alternatives is expected to bolster market growth.
- One of the major Europe intracranial aneurysm market trends is the increased strategic growth initiatives by the key market players to boost their presence in the European market. In January 2024, Evasc Neurovascular announced expansion plans for its neurovascular treatment portfolio in the European market through the establishment of a German subsidiary, Evasc Neurovascular GmbH.
- Increased access to novel devices in the region is expected to lead to higher adoption rates in the healthcare sector and fuel market demand. Nautilus intrasaccular system, developed by Endostream Medical was indicated as the “next evolution” in aneurysm care at the European Society of Minimally Invasive Neurological Therapy (ESMINT), conducted in September 2023, the. The intrasaccular flow diverter, intended for the treatment of intracranial aneurysms, is CE-marked and is available in the European market.
Europe Intracranial Aneurysm Market Analysis
Intracranial aneurysm or brain aneurysm usually occurs at the underside of the brain and the base of the skull and is characterized by ballooning or bulging in a blood vessel in the brain. The condition can affect anyone at any age, but they are more common in people aged 40 or older. Additionally, women are more susceptible to develop the condition as compared to men. The rising advancements in treatment options and diagnostic techniques are resulting in increased treatment-seeking behavior and early diagnosis rates, which is expected to drive Europe intracranial aneurysm market growth in the coming years.When an intracranial aneurysm ruptures, bleeding occurs in the tissue surrounding the brain. The condition is fatal in around 50% of cases of ruptured brain aneurysms. Recent data suggests that about 25% of people die within the first 24 hours of the rupture and nearly 50% die within 3 months . This indicates the immediate need for effective treatment alternatives that can tackle the rising cases of mortality associated with ruptured aneurysms in the brain. Further, increased investment in the development of innovative diagnostic techniques is expected to lead to early diagnosis and improve patient outcomes. Consequently, this is poised to fuel Europe intracranial aneurysm market demand in the forecast period.
Europe has a large patient pool affected by intracranial aneurysms owing to the increasing geriatric population more prone to the condition. The rising prevalence of the disease makes Europe a lucrative market, attracting investments from the leading market players. For example, in January 2024, Evasc Neurovascular, a medical device company specializing in endovascular repair of cerebral aneurysms, announced strategic growth initiatives that involved the expansion plans of its neurovascular treatment portfolio in the European market through the establishment of a German subsidiary, Evasc Neurovascular GmbH. The company which recently boasted 120 successful intracranial aneurysm cases with its novel Eclips device is expected to advance neurovascular care in the region. Thus, the strong presence of key market players not only improves access to treatment options but also significantly contributes to augmenting Europe intracranial aneurysm market value.
The market is witnessing a steady development and commercialization of innovative endovascular techniques and devices, leading to higher adoption rates of these safer and more effective alternatives. In September 2023, the Nautilus intrasaccular system developed by Endostream Medical (a company developing solutions for brain aneurysms) was indicated as the “next evolution” in aneurysm care at the European Society of Minimally Invasive Neurological Therapy (ESMINT). The intrasaccular flow diverter, intended for the treatment of intracranial aneurysms, is CE-marked and is available in the European market. The increased accessibility to such novel devices is expected to result in faster recovery times and reduced morbidity and mortality rates, thereby propelling market growth in the region.
Europe Intracranial Aneurysm Market Segmentation
Market Breakup by Treatment Type
- Endovascular Coiling
- Surgical Clipping
- Flow Diverters
- Other Treatment Types
Market Breakup by End User
- Hospitals
- Clinics
- Others
Market Breakup by Countries
- United Kingdom
- Germany
- France
- Italy
Europe Intracranial Aneurysm Market: Competitor Landscape
The key features of the market report include patent analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Medtronic
- Stryker
- MicroPort Scientific Corporation
- Johnson & Johnson Services, Inc
- MicroVention Inc
- Integra LifeSciences
- B. Braun Melsungen AG
- Delta Surgical
- Mizuho Medical Co. Ltd.
- Penumbra, Inc
FAQs
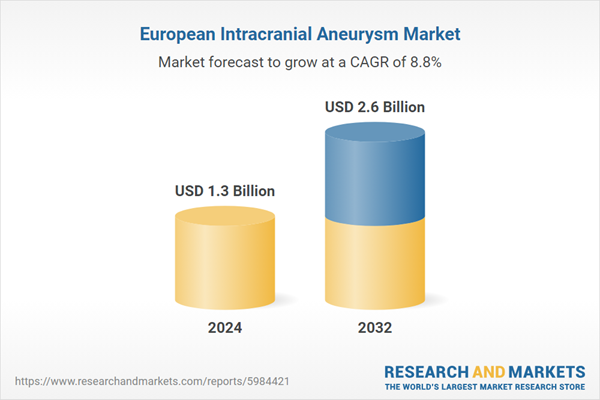
What is the Europe intracranial aneurysm market forecast outlook for 2024-2032?The Europe intracranial aneurysm market is expected to be driven by the rising demand in the global market, which is anticipated to grow at a CAGR of 8.8% during the forecast period of 2024-2032 and is likely to reach a market value of USD 2.6 billion by 2032.
What are the major factors aiding the Europe intracranial aneurysm market demand?
The growing demand for minimally invasive procedures and the rising healthcare expenditure are fuelling the demand for the market.
What are the major Europe intracranial aneurysm market trends?
One of the significant trends in the market is the increased strategic growth initiatives by the key market players to boost their presence in the European market. In January 2024, Evasc Neurovascular announced its expansion plans for its neurovascular treatment portfolio in the European market through the establishment of a German subsidiary, Evasc Neurovascular GmbH.
What is the market segmentation based on the treatment type?
Based on the treatment type, the market is segmented into endovascular coiling, surgical clipping, and flow diverters, among other treatment types.
What are the major end users of the market?
End users of the market are hospitals and clinics, among other end-users.
What is the market segmentation by countries?
The market segmentation by countries includes the United Kingdom, Germany, France, and Italy, among others.
Who are the key players involved in the Europe intracranial aneurysm market?
The key players in the market are Medtronic, Stryker, MicroPort Scientific Corporation, Johnson & Johnson Services, Inc., MicroVention Inc., Integra LifeSciences, B. Braun Melsungen AG, Delta Surgical, Mizuho Medical Co. Ltd., and Penumbra, Inc.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Medtronic
- Stryker
- MicroPort Scientific Corporation
- Johnson & Johnson Services Inc
- MicroVention Inc
- Integra LifeSciences
- B. Braun Melsungen AG
- Delta Surgical
- Mizuho Medical Co. Ltd.
- Penumbra Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | June 2024 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 2.6 Billion |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 10 |