Growing Incidence of Stroke and Musculoskeletal Drives Europe Medical Exoskeleton Market
Despite the advancements in the medical industry, stroke continues to be a major public health issue. Stroke ranks second in the world in terms of causes of mortality and is the main cause of disability. According to the Global Stroke Factsheet 2022, the lifetime risk of having a stroke has risen by 50% in the past 17 years, with an estimated 1 in 4 people expected to experience one at some point in their lives. Between 1990 and 2019, there was a rise of 70% in the incidence of stroke, 43% in the number of stroke-related deaths, 102% in the frequency of stroke, and 143% in Disability Adjusted Life Years (DALY).The most notable aspect is that lower- and lower-middle-income countries account for the majority of the world's stroke burden (86% of stroke-related fatalities and 89% of DALYs). In the European Union, there were 1.12 million stroke incidents, 9.53 million stroke survivors, 0.46 million stroke-related deaths, and 7.06 million years of life lost due to disability from stroke in 2017.
Further, the number of people living with stroke is predicted to rise by 27% between 2017 and 2047, primarily as a result of aging populations and higher survival rates. It is anticipated that differences will continue to exist between nations, indicating potential for advancements in case management and prevention, especially in Eastern Europe. Therefore, the increasing incidences of stroke have boosted the demand for exoskeleton systems for rehabilitation of stroke patients to help improve the quality of life.
In addition, musculoskeletal disorders impact the mobility of an individual. The increasing prevalence of musculoskeletal disorders, such as tendinitis, osteopenia, sarcopenia, and low back injuries, result in a growing need for exoskeletan robotic systems. According to the World Health Organization (WHO), ~1.71 billion people globally are affected by musculoskeletal diseases, and lower back pain is a leading cause of disability in 160 countries. These exoskeletons assist patients in managing their pain, as well as help regain their mobility, thereby improving their overall quality of life. Thus, the increasing prevalence of stroke and musculoskeletal disorders has led to the use of exoskeletan robotic systems for rehabilitation.
Europe Medical Exoskeleton Market Overview
The European medical exoskeleton market is segmented into Germany, the UK, France, Italy, Spain, and the Rest of Europe. The region holds a significant share of the global medical exoskeleton market and is expected to register a notable CAGR. Market players in the country are adopting inorganic growth strategies such as partnerships and collaborations for market expansion and growth.In August 2022, German Bionic (a global technology leader in the development of smart power suits) and Mubea (the top German automotive supplier) entered a cooperation agreement. By assisting the world's top developer of robotic exoskeletons in production, Mubea tapped into a new and quite promising future sector. Similarly, in April 2020, ReWalk Robotics Ltd.
announced the national agreement with top German Statutory Health Insurers ("SHI"), Deutsche Angestellten-Krankenkasse - Gesundheit ("DAK-Gesundheit"), and Techniker Krankenkasse ("TK"). This agreement can permit any eligible beneficiary with a spinal cord injury to get a ReWalk 6.0 exoskeleton system. TK and DAK, representing 10.6 million and 5.6 million beneficiaries in Germany, respectively, are two of the largest SHIs in the country; these agreements are expected to help set the standard for exoskeleton procurement in Germany.
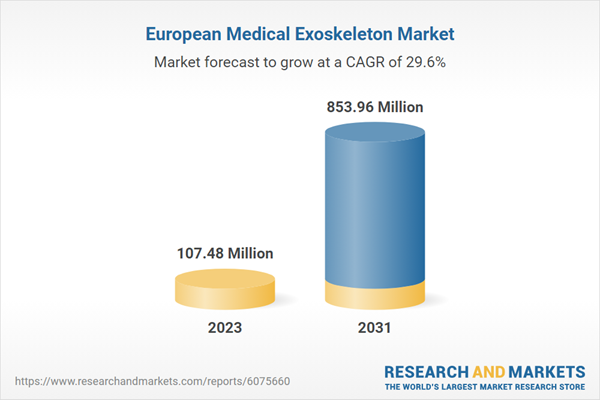
Europe Medical Exoskeleton Market Revenue and Forecast to 2031 (US$ Million)
Europe Medical Exoskeleton Market Segmentation
The Europe medical exoskeleton market is categorized into component, type, extremity, application, mobility, end users, and country.By component, the Europe medical exoskeleton market is bifurcated into hardware and software. The hardware segment held a larger share of the Europe medical exoskeleton market share in 2023.
In terms of type, the Europe medical exoskeleton market is bifurcated into powered exoskeleton and passive exoskeleton. The powered exoskeleton segment held a larger share of the Europe medical exoskeleton market share in 2023.
Based on extremity, the Europe medical exoskeleton market is segmented into lower body exoskeleton, upper body exoskeleton, and full body exoskeleton. The lower body exoskeleton segment held the largest share of the Europe medical exoskeleton market share in 2023.
By application, the Europe medical exoskeleton market is segmented into spinal cord injury, multiple sclerosis, stroke, cerebral palsy, Parkinson’s Disease, and others. The spinal cord injury segment held the largest share of the Europe medical exoskeleton market share in 2023.
In terms of mobility, the Europe medical exoskeleton market is bifurcated into mobile exoskeleton and stationary exoskeleton. The powered exoskeleton segment held a larger share of the Europe medical exoskeleton market share in 2023.
By end users, the Europe medical exoskeleton market is segmented into rehabilitation centers, physiotherapy centers, long term care centers, homecare settings, and others. The rehabilitation centers segment held the largest share of the Europe medical exoskeleton market share in 2023.
Based on country, the Europe medical exoskeleton market is segmented into Germany, France, the UK, Italy, Spain, and the Rest of Europe. Germany segment held the largest share of Europe medical exoskeleton market in 2023.
BIONIK; B-Temia Inc; Cyberdyne Inc; Ekso Bionics Holdings Inc; ExoAtlet; Hocoma AG; Lifeward, Inc; Myomo Inc; Rex Bionics Ltd.; and Wearable Robotics SR are some of the leading companies operating in the Europe medical exoskeleton market.
Reasons to buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Europe medical exoskeleton market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the Europe medical exoskeleton market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth Europe market trends and outlook coupled with the factors driving the Europe medical exoskeleton market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution.
Table of Contents
Companies Mentioned
Some of the leading companies in the Europe Medical Exoskeleton Market include:- Ekso Bionics Holdings Inc
- Lifeward, Inc
- ExoAtlet
- Cyberdyne Inc
- BIONIK
- B-Temia Inc
- Hocoma AG
- Wearable Robotics SRL
- Myomo Inc
- Rex Bionics Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 117 |
| Published | February 2025 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 107.48 Million |
| Forecasted Market Value ( USD | $ 853.96 Million |
| Compound Annual Growth Rate | 29.6% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 11 |