Speak directly to the analyst to clarify any post sales queries you may have.
A focused orientation that defines the report scope, clarifies stakeholder priorities, aligns clinical and procurement objectives, and frames the analytical approach for decision-makers
This introduction clarifies the purpose, scope, and audience for a comprehensive examination of external defibrillators, setting expectations for how findings translate into operational and strategic action. It outlines the critical questions addressed across technology, clinical application, end-user deployment, and supply channels, while framing safety and regulatory compliance as primary drivers of procurement decisions. Readers are oriented to the kinds of evidence used, the balance between clinical, commercial, and policy perspectives, and the decision points most relevant to hospital administrators, public safety planners, device manufacturers, and institutional buyers.
The narrative establishes a common language for subsequent sections so that technical terms, deployment models, and end-user needs remain consistent and comparable. It also highlights the interplay between innovation in device design and practical considerations such as training, servicing, and point-of-care integration. By clarifying these dimensions up front, the introduction ensures that subsequent analysis remains actionable and centered on improving survival outcomes, optimizing total cost of ownership, and aligning procurement choices with real-world operational constraints.
An analysis of how technological integration, regulatory evolution, and shifting end-user expectations are reshaping procurement priorities and competitive advantage in lifesaving devices
The external defibrillator landscape is undergoing multiple transformative shifts driven by technological convergence, regulatory emphasis on public access to life-saving devices, and a renewed focus on post-acute care continuity. Emerging software and connectivity capabilities are turning standalone hardware into system elements within broader emergency response networks, enabling real-time monitoring, predictive maintenance, and integration with emergency medical services. At the same time, end-user expectations have evolved: purchasers now weigh usability, telemetry, and integrated training ecosystems as heavily as device reliability.
Concurrently, clinical practice is shaping device specifications through greater emphasis on pediatric-ready algorithms and ergonomics for non-clinical responders. Regulatory agencies and procurement bodies have elevated standards for device labeling, interoperability, and maintenance documentation, which in turn prompts manufacturers to adopt robust quality systems and digital traceability. These dynamics collectively accelerate differentiation around after-sales services, software-enabled features, and strategic partnerships with healthcare providers and public agencies, shifting competitive advantage from purely hardware excellence to integrated system capability.
A practical examination of how recent tariff measures are reshaping sourcing, contractual protections, and supply chain resilience strategies across device manufacturing and distribution
Policy adjustments affecting tariffs can ripple across supply chains, procurement timing, and product configuration decisions without necessarily altering clinical effectiveness. Recent tariff actions have increased attention to sourcing strategies, with organizations reevaluating where component manufacturing, final assembly, and software development occur. In response, procurement teams are conducting more granular supplier risk assessments and revising total cost frameworks to incorporate potential tariff volatility, customs clearance complexity, and the administrative burden of cross-border logistics.
Manufacturers and distributors have begun to reassess manufacturing footprints, exploring dual-sourcing, nearshoring, or localized assembly to mitigate exposure. These operational shifts influence lead times, spare-part availability, and the structure of service contracts. Meanwhile, public agencies and large institutional buyers are updating procurement language to include tariff contingency clauses and to demand supply chain transparency. Consequently, procurement cycles may lengthen as stakeholders seek contractual protections and suppliers reassess pricing strategies, but buyers benefit from clearer accountability and improved resilience through diversified sourcing and stronger contractual guarantees.
Deep segmentation insights that illuminate how product type, technology choice, patient profile, end-user environment, and supply channel drive adoption dynamics and procurement criteria
Segment-level dynamics reveal distinct adoption patterns and procurement drivers across product, technology, patient, end-user, and supply-channel dimensions. Product segmentation differentiates Automated External Defibrillators from Manual External Defibrillators, with further detail recognizing Fully Automated AED options and Semi-Automated AED variants that cater to different responder skill levels and use cases. Technology segmentation highlights the operational implications of Biphasic Technology versus Monophasic Technology, influencing energy delivery profiles and compatibility with clinician protocols. Patient-type segmentation distinguishes adult from pediatric applications, with pediatric readiness requiring algorithmic adjustments, pediatric pads, and training considerations to enable safe usage across age groups.
End-user segmentation exhibits the broadest variance in procurement criteria: Home care settings emphasize ease of use and compact form factors, with sub-focus areas for elderly care and post-operative care that prioritize reliability and caregiver training. Hospital procurement differentiates by clinical area, where cardiac units demand advanced diagnostics and integration capabilities, emergency departments require rapid accessibility and robustness, and general wards balance cost with basic resuscitation readiness. Public access deployments concentrate on visibility, signage, and vandal-resistant packaging across environments such as airports, shopping malls, sports facilities, and transport stations. Finally, supply-channel segmentation distinguishes between offline retail and online platforms, each presenting different warranty, fulfillment, and education-touchpoint models that influence buyer confidence and post-sale support strategies.
Key regional perspectives that contrast procurement priorities, regulatory frameworks, and deployment models across major global markets to inform market entry and scale strategies
Regional dynamics vary substantially, with distinct regulatory environments, procurement cultures, and deployment priorities shaping adoption and operational models. In the Americas, a strong emphasis on public access initiatives and integrated emergency response systems has driven collaborations among health systems, municipal planners, and private-sector suppliers. This region places particular focus on interoperability with EMS dispatch systems and on robust training programs that empower lay responders.
In Europe, Middle East & Africa, regulatory harmonization efforts coexist with heterogeneous procurement appetites; some markets emphasize centralized hospital procurement and clinical integration, while others prioritize public access programs and decentralized deployment. Sellers must navigate diverse certification regimes and establish localized support networks to ensure uptime and compliance. In Asia-Pacific, rapid urbanization and growing private healthcare investment are increasing demand for both institutional and public-access deployments. This region also shows a rising interest in compact, cost-effective devices paired with digital training and telemedicine integration to support broader geographic coverage and remote maintenance models.
Competitive intelligence highlighting how product innovation, integrated services, and distribution excellence determine leadership and enable sustainable commercial models in the device market
Competitive dynamics center on product differentiation through integrated services, software-enabled features, regulatory compliance rigor, and strategic partnerships with clinical networks. Leading manufacturers are investing in user-centered design, intuitive interfaces, and connectivity that supports remote status monitoring and automated maintenance workflows. These capabilities support recurring revenue models tied to consumables and service agreements, reinforcing long-term relationships with institutional buyers.
At the same time, a cohort of agile firms focuses on niche opportunities, offering compact, cost-optimized devices for home care and targeted public access deployments. Partnerships between device makers and training providers are becoming commonplace, enabling bundled offerings that align equipment procurement with responder readiness. Distribution strategies are also evolving: channel partners and third-party logistics firms that can guarantee rapid replacement parts, certified servicing, and localized technical support gain competitive advantage. Collectively, these trends favor players that combine robust product quality with ecosystem services and transparent regulatory documentation.
Actionable recommendations that align product-service integration, procurement safeguards, and resilient sourcing to protect device readiness and improve clinical outcomes
Industry leaders should prioritize integrated product-service packages that pair robust hardware with software-enabled maintenance and evidence-based training, thereby reducing total operational risk for buyers. Investing in interoperable telemetry and predictive maintenance platforms helps preserve device readiness and creates new value propositions for recurring service revenue. To operationalize this, manufacturers can phase deployments of connected features and validate value through pilot programs with diverse end users, then scale successful models across broader customer segments.
Buyers, including hospitals and public agencies, should insist on contractual transparency around warranty, spare-part guarantees, and tariff contingency. They should also evaluate suppliers on their ability to provide localized servicing and rapid replacement logistics. Procurement teams will benefit from specifying pediatric-ready capabilities where appropriate and from embedding training commitments in purchase agreements to ensure on-site competence. Strategic collaboration between manufacturers and major buyers on training curricula and incident reporting can improve outcomes and reduce the incidence of device downtime.
Finally, stakeholders should adopt flexible sourcing strategies that balance cost efficiency with resilience. Dual-sourcing, localized assembly, and inventory buffers for critical consumables reduce exposure to policy-driven disruptions. Through these measures, organizations can secure device availability while preserving budgetary discipline and clinical readiness.
A transparent, mixed-methods research approach combining primary stakeholder interviews, document analysis, supply chain validation, and scenario testing to produce operationally relevant intelligence
The research approach combined primary qualitative interviews, secondary document analysis, and iterative validation to ensure robust, decision-ready insights. Primary research involved structured discussions with clinicians, procurement leaders, emergency response coordinators, and device maintenance specialists to capture first-hand operational challenges and priorities. Secondary research examined regulatory publications, technical standards, and product documentation to establish the factual baseline for technology and compliance considerations. These inputs were triangulated to reconcile differing perspectives and to surface actionable implications.
Data integrity was further reinforced through cross-validation with supply chain partners and training providers to confirm service-level realities and aftermarket dynamics. The methodology also employed scenario-based analysis to test how policy changes, such as tariff adjustments, could affect sourcing and procurement behavior. Throughout the process, emphasis remained on qualitative depth and operational relevance rather than on speculative numerical forecasting, ensuring that findings are practical for decision-makers weighing procurement, deployment, and training options.
A concise synthesis underscoring that device effectiveness depends equally on operational readiness, supply chain transparency, and integrated training to improve emergency outcomes
In closing, the report synthesizes how technological progress, regulatory expectations, and evolving end-user needs converge to redefine the landscape for external defibrillators. Stakeholders that adapt through integrated product-service offerings, resilient sourcing arrangements, and clear training and maintenance commitments will be best positioned to improve device availability and patient outcomes. The analysis emphasizes that clinical efficacy alone is insufficient; operational readiness, supply chain transparency, and user competence are equally critical to realizing the lifesaving potential of deployed devices.
Decision-makers should therefore prioritize investments that align with their operational constraints and risk tolerance, engage suppliers who can demonstrate comprehensive support capabilities, and embed performance and maintenance clauses into procurement contracts. By doing so, institutions can convert technological advances into measurable improvements in emergency response and continuity of care, while shielding their operations from policy and supply-side volatility.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China External Defibrillators Market
Companies Mentioned
The key companies profiled in this External Defibrillators market report include:- A.M.I. Italia S.r.l.
- AED Solutions Inc.
- Asahi Kasei Corporation
- Bioevopeak Co., Ltd.
- BPL Medical Technologies Private Limited
- CardioComm Solutions, Inc.
- Cu Medical Germany GmbH
- Defibtech, Inc.
- Dixion Vertrieb medizinischer Geräte GmbH
- EMS Mobil Sistemler A.Ş.
- General Electric Company
- GS Elektromedizinische Geräte G. Stemple GmbH
- Koninklijke Philips N.V.
- Laerdal Medical AS
- Mediana Co., Ltd.
- Metsis Medikal Ltd.
- Mindray Medical International Ltd.
- Nihon Kohden Corporation
- OSI Systems, Inc.
- Progetti S.r.l.
- Resuscitation Systems LLC
- Schiller AG
- Stryker Corporation
- Zoll Medical Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
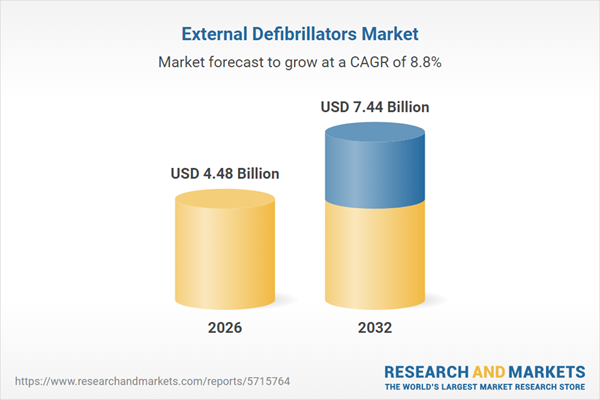
| Estimated Market Value ( USD | $ 4.48 Billion |
| Forecasted Market Value ( USD | $ 7.44 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |