Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive foundational overview of febantel’s therapeutic role, clinical utility, regulatory interactions, supply chain influences and stewardship implications in veterinary care
Febantel occupies a distinct and consequential position within veterinary parasitology as an anthelmintic employed across both companion and farm animal care. Its mechanism, which effectively targets a spectrum of gastrointestinal nematodes when administered as a prodrug in suitable formulations, underpins its continued clinical utility. Practitioners favor febantel for integrated parasite control protocols, often leveraging its synergistic profile when combined with agents such as praziquantel or pyrantel to broaden efficacy against mixed parasitic burdens. Consequently, febantel remains relevant across a variety of treatment paradigms that prioritize safety, palatability, and ease of administration.Regulatory evolution and advancing clinical practice shape how febantel is manufactured, formulated, and distributed. Contemporary expectations emphasize rigorous residue testing for food-producing animals, validated stability for multi-dose presentations, and clear labeling that supports responsible use in companion species. Parallel to clinical drivers, supply chain dynamics and ingredient sourcing influence product continuity and cost structures. As stewardship programs intensify and diagnostic capabilities improve, febantel’s role will continue to be calibrated against resistance monitoring outcomes and the need for integrated parasite management approaches that combine prophylactic strategies, targeted treatments, and owner education to sustain therapeutic effectiveness.
Identification and analysis of the major transformative forces redefining febantel development, distribution, stewardship, and clinical adoption across animal health markets
Multiple converging forces are reshaping how febantel is developed, delivered, and clinically adopted across veterinary markets. Advances in diagnostic precision enable clinicians to move from blanket treatment approaches toward targeted, evidence-based deworming schedules that prioritize efficacy while reducing unnecessary exposure. This shift elevates demand for formulations that support compliance and accurate dosing, including palatable tablets and ready-to-administer liquids suitable for both individual and herd applications. At the same time, heightened emphasis on antimicrobial and anthelmintic stewardship has driven adoption of combination therapies to simplify protocols and enhance spectrum of activity, while also necessitating robust post-marketing surveillance to detect emerging resistance patterns.Supply chain diversification and digital distribution are altering commercial dynamics. Manufacturers and contract producers increasingly explore geographic sourcing alternatives and flexible manufacturing arrangements to mitigate raw material volatility. Digital channels and direct-to-consumer ecommerce platforms expand access to products previously confined to clinic shelves, encouraging innovation in packaging and patient adherence tools. Regulatory frameworks are also adapting, with authorities increasingly focused on residue limits, veterinary oversight, and traceability, prompting companies to strengthen their regulatory dossiers and invest in data that demonstrates product safety and performance across varied animal populations. Together, these shifts create both risk and opportunity for stakeholders seeking to maintain therapeutic relevance while responding to an evolving health ecosystem.
Analytical synthesis of the cumulative commercial and operational implications stemming from the United States tariff measures introduced in 2025 and their ripple effects across the febantel supply chain
The introduction of new tariff measures in 2025 has prompted stakeholders across the febantel value chain to reassess sourcing, inventory, and pricing strategies. Import duties and associated trade compliance requirements increase the landed cost of active pharmaceutical ingredients and finished formulations when sourced from jurisdictions subject to the tariffs. This pressure has encouraged distributors and manufacturers to explore alternative sourcing corridors, consolidate purchases to achieve scale, and renegotiate supply agreements to preserve margin structures. For some producers, tariff-related cost shifts have triggered a re-evaluation of nearshoring or establishing additional contract manufacturing partnerships to shorten supply lines and reduce exposure to cross-border policy volatility.Commercial actors have responded with pragmatic operational adjustments aimed at maintaining product availability and clinical continuity. Inventory management practices have tightened, with companies adopting scenario planning to balance stockholding costs against the risk of supply interruptions. Pricing strategies have adjusted incrementally in markets where regulatory frameworks permit pass-through of increased acquisition costs, while in other regions companies have absorbed portions of the impact to sustain volume and preserve relationships with veterinary customers. Overall, the tariff environment has accelerated strategic conversations about supply resilience, regulatory compliance for alternate sourcing, and the value of diversifying distribution channels to mitigate concentrated exposure to affected trade lanes.
In-depth segmentation-driven insights revealing how animal type, distribution channels, formulation variants, and combination product strategies shape febantel clinical adoption and commercialization approaches
A granular view of febantel demand and product performance is best understood through its primary segmentation lenses, which reveal distinct clinical and commercial patterns across animal types, distribution channels, formulations, and product archetypes. Within animal type, companion animals such as cats and dogs often drive demand for convenient dosing formats that prioritize palatability and owner adherence; film-coated tablets and user-friendly liquids support treatment regimens administered at home and align with preventive care conversations in clinical settings. In contrast, farm animals including cattle, pigs, poultry, and sheep typically require high-volume, cost-effective presentations and formulations that facilitate group administration, dosing accuracy, and residue management for food-safety compliance.Distribution channel dynamics further influence product design and go-to-market approaches. Online retailers, encompassing both direct ecommerce storefronts and third-party marketplaces, expand reach to pet owners seeking convenience, prompting manufacturers to consider consumer-pack sizes and fulfillment reliability. Pharmacies continue to serve as a trusted retail option for over-the-counter and prescription offerings, while veterinary clinics remain pivotal for professional recommendation and adherence counseling, especially for complex combination therapies. Formulation choices-liquids available as solutions or suspensions, powders, and tablets in film-coated or uncoated forms-must reflect the intended use case, stability needs, and dosing precision for both individual and herd applications. Product type distinctions between combination therapies and monotherapies also shape clinical positioning; combinations pairing febantel with agents such as praziquantel or pyrantel address multi-parasite burdens with a single protocol, which can improve compliance and reduce clinic time but introduces additional regulatory and bioequivalence considerations. By mapping these segments against clinical workflows and commercial channels, stakeholders can better align product portfolios with end-user needs and operational constraints.
Strategic regional analysis that clarifies how regulatory regimes, clinical practices, and distribution landscapes across the Americas, Europe Middle East & Africa, and Asia-Pacific influence febantel utilization and supply choices
Regional dynamics exert a pronounced influence on febantel usage patterns, regulatory priorities, and commercial strategies across the Americas, Europe Middle East & Africa, and Asia-Pacific territories. In the Americas, companion animal veterinary services and direct-to-consumer engagement have gained momentum, encouraging manufacturers to tailor formulations that emphasize convenience and owner compliance and to invest in educational campaigns that support responsible parasite management. Regulatory approaches in this region focus on veterinary oversight and safety labeling, and distribution ecosystems increasingly leverage online sales to meet consumer demand for accessible products.Across Europe, the Middle East, and Africa, regulatory rigor and residue control remain paramount, particularly for food-producing species, driving comprehensive testing and documentation requirements for formulations intended for cattle, sheep, poultry, and swine. Market access strategies in these areas often emphasize well-documented safety profiles and resilient supply chains to satisfy importers and regulators. In the Asia-Pacific region, a mix of large-scale livestock operations and rapidly expanding companion animal ownership shapes demand; competitive pricing pressures coexist with significant local manufacturing capabilities. Manufacturers operating in this region frequently pursue localized production and registration pathways to meet volume needs and navigate diverse regulatory regimes. Each regional landscape requires tailored commercial and regulatory approaches that reflect prevailing clinical practices, distribution infrastructure, and policy priorities.
Key corporate and competitive insights detailing how product innovators, generics manufacturers, and contract producers influence febantel availability, formulation innovation, and regulatory positioning across markets
The febantel ecosystem features a blend of established multinational animal health companies, specialized generics manufacturers, and an expanding network of contract development and manufacturing organizations. Market incumbents typically leverage broad regulatory experience and established distribution partnerships to expedite registration and sustain product availability, while generics and specialty firms focus on cost-efficiency, niche formulations, and rapid time-to-market for off-patent combinations. Contract manufacturers play a pivotal role in scaling production and providing geographic redundancy, particularly when tariff shifts and raw material disruptions necessitate agile capacity adjustments.Competitive dynamics are further shaped by investment in formulation innovation, quality assurance systems, and robust regulatory dossiers that facilitate multi-jurisdictional approvals. Strategic partnerships between product developers and diagnostic or digital veterinary service providers foster integrated solutions that pair therapeutics with adherence tools and treatment monitoring. Intellectual property considerations for combination products, alongside the need for clear bioequivalence and safety data, influence go-to-market timing and collaboration choices. Companies that prioritize supply reliability, transparent quality practices, and proactive regulatory engagement tend to preserve customer trust and sustain long-term commercial relationships with veterinary professionals and distributors.
Actionable strategic recommendations for manufacturers and distributors to bolster febantel supply resilience, regulatory readiness, channel expansion, and product differentiation in evolving markets
Leaders seeking to strengthen their position in febantel therapeutics should adopt a multi-pronged strategy that balances supply resilience, regulatory rigor, and end-user centricity. Prioritizing geographic diversification of active ingredient and finished product sourcing reduces exposure to trade disruptions and allows for faster operational pivots when policy changes occur. Investing in robust quality systems, clear residue data, and comprehensive regulatory dossiers streamlines multi-market registrations and supports confidence among regulatory authorities and veterinary customers. Simultaneously, developing combination products and patient-friendly formulations can enhance clinical uptake while meeting stewardship expectations, provided these products are backed by clear safety and bioequivalence evidence.Commercially, enhancing digital channel capabilities and strengthening relationships with veterinary professionals will support sustained demand and improve treatment adherence. Scenario planning and dynamic inventory strategies mitigate the operational impact of tariff volatility or supply interruptions. Collaborating with contract manufacturers and third-party logistics providers can expand capacity without large capital outlays, while strategic alliances with diagnostic and service providers offer differentiated value propositions to clinicians. Finally, transparent communication around responsible use, residue management, and efficacy builds trust with both regulators and end-users and positions companies to capitalize on long-term clinical and commercial opportunities.
Transparent explanation of the mixed-methods research approach combining primary stakeholder interviews, regulatory and trade analysis, patent and formulation review, and scenario triangulation to generate robust insights
This analysis synthesizes evidence gathered through a mixed-methods research approach designed to capture both macro-level trade dynamics and micro-level clinical preferences. The methodology incorporated a structured review of peer-reviewed literature, regulatory guidance documents, patent filings, and public trade data to map supply chain contours and policy changes. Primary qualitative research included interviews with practicing veterinarians, formulation scientists, regulatory affairs specialists, distribution executives, and contract manufacturing representatives to validate assumptions around clinical use, packaging preferences, and logistical constraints.Analytical triangulation was employed to reconcile differences between primary insights and public data, ensuring robust interpretation of emerging trends. Segmentation mapping translated clinical and channel requirements into actionable product and go-to-market implications, while scenario analysis examined the operational impacts of tariff adjustments and supply interruptions. The research team applied evidence-weighting to prioritize high-confidence findings and identified areas where additional primary data collection would enhance precision for tactical decision-making.
Concise and authoritative conclusion that synthesizes clinical value, supply resilience needs, regulatory considerations, and commercial imperatives shaping febantel strategies across veterinary markets
The synthesis of clinical, commercial, and policy perspectives underscores febantel’s continued relevance as a flexible antiparasitic option across diverse veterinary contexts. Its clinical value is strengthened when integrated into thoughtfully designed treatment regimens that reflect diagnostic guidance, animal type-specific needs, and stewardship commitments. Market and operational pressures, including tariff-driven cost shifts and changing distribution behaviors, necessitate proactive strategies around sourcing, regulatory documentation, and customer engagement to preserve availability and clinical utility.Decision-makers who align formulation innovation, supply resiliency, and regulatory readiness with practical distribution models will be best positioned to meet practitioner expectations and adapt to policy changes. By combining evidence-driven product design with agile commercial execution, stakeholders can sustain febantel’s therapeutic contributions while addressing the operational challenges that characterize today’s animal health landscape.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Febantel Market
Companies Mentioned
- Alivira Animal Health Limited
- Angle Bio Pharma
- Jigs Chemical Limited
- Niksan Pharmaceutical
- Omshri Labs Pvt Ltd.
- Pranshav Health Care
- Pure Chem Pvt. Ltd.
- Raxuter Chemicals
- Salvavidas Pharmaceutical Pvt. Ltd.
- Shreeji Pharma International
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
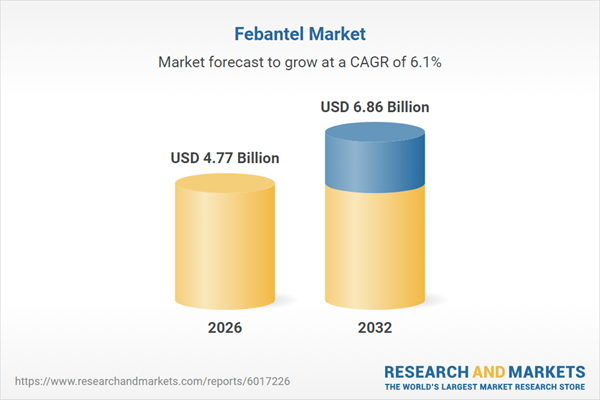
| Estimated Market Value ( USD | $ 4.77 Billion |
| Forecasted Market Value ( USD | $ 6.86 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |