This new report “France Diabetes Market Report: 2024-2032” provides an analytical and statistical insight into the France diabetes market. The report provides both current and future trends in the prevalence, demographical breakup, diagnosis and treatment of diabetes in France. The research study serves as an exceptional tool to understand the epidemiology, market trends, therapeutic structure, competitive structure and the outlook of the France diabetes market. This report can serve as an excellent guide for investors, researchers, consultants, marketing strategists and all those who are planning to foray into the France diabetes market in any form.
What this Report Achieves:
Comprehensive situation analysis of the France diabetes epidemiology and its dynamics:
Focus of the Analysis:
Historical, current and future prevalence of diabetes in FranceHistorical, current and future prevalence of type-1 and type-2 diabetes in France
Historical, current and future prevalence of diabetes in the urban and rural regions in France
Historical, current and future prevalence of diabetes among males and females in France
Historical, current and future prevalence of diabetes among various age groups in France
Historical, current and future diagnosis rates for diabetes in France
Historical, current and future drug treatment rates for diabetes in France
Comprehensive situation analysis of the France Oral Antidiabetics market and its dynamics:
Focus of the Analysis:
Performance of the Oral Antidiabetics market in FrancePerformance of key classes
Performance of key players
Market outlook
Comprehensive situation analysis of the France Insulin market and its dynamics:
Focus of the Analysis:
Performance of the Insulin market in FrancePerformance of key classes
Performance of key players
Market outlook
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | July 2024 |
| Forecast Period | 2023 - 2032 |
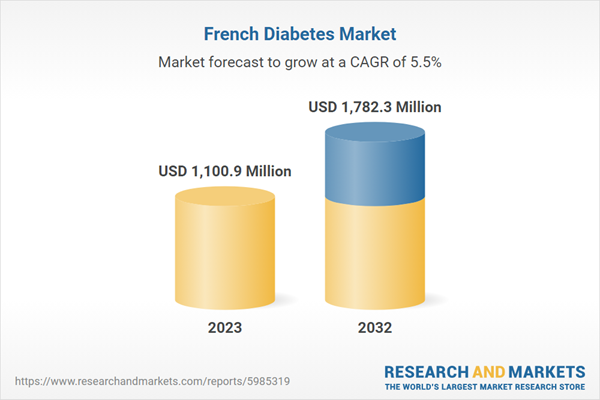
| Estimated Market Value ( USD | $ 1100.9 Million |
| Forecasted Market Value ( USD | $ 1782.3 Million |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | France |