Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Aging Population
France, like many developed nations, is witnessing a demographic transformation characterized by an increasingly aging population. This demographic shift not only poses challenges for healthcare systems but also presents unique opportunities for specific medical sectors. One such area experiencing a significant upswing is the France Bone Void Fillers Market.As individuals age, the likelihood of encountering orthopedic issues such as fractures, degenerative bone diseases, and osteoporosis rises. The aging process itself contributes to a decrease in bone density and an increased susceptibility to fractures. Bone void fillers emerge as crucial solutions in addressing these conditions, stimulating bone regeneration, and facilitating the healing process.
Osteoporosis, a condition characterized by weakened bones, is prevalent among the elderly population. With bones becoming more susceptible to fractures, the demand for interventions like bone void fillers rises. These fillers not only provide structural support but also aid in restoring bone density, offering a viable solution for those grappling with the effects of osteoporosis.
The aging population often expresses a preference for non-invasive or minimally invasive treatments over traditional surgical procedures. Bone void fillers, being a minimally invasive option, align with this preference. This demographic cohort seeks interventions that not only effectively address their orthopedic issues but also offer quicker recovery times and reduced postoperative complications.
Joint replacement surgeries, often necessitated by age-related conditions such as arthritis, contribute to the demand for bone void fillers. These fillers play a crucial role in enhancing the success of joint replacement procedures by providing support and aiding in the integration of implants. The aging population's inclination towards maintaining an active lifestyle further propels the need for such joint-preserving interventions.
The healthcare system in France is adapting to the changing demographic landscape, recognizing the unique healthcare needs of the elderly. This adaptation includes the incorporation of advanced orthopedic solutions like bone void fillers into treatment protocols. Healthcare providers are increasingly integrating these interventions into their offerings to cater to the specific requirements of an aging patient demographic.
Increasing Prevalence of Orthopedic Disorders
France, like many developed nations, is witnessing a notable surge in the prevalence of orthopedic disorders. This increase, attributed to various factors including lifestyle changes and an aging population, is reshaping the landscape of orthopedic care. Within this evolving scenario, the France Bone Void Fillers Market emerges as a key player, providing innovative solutions to address the growing incidence of orthopedic issues.Orthopedic disorders often manifest in the form of fractures and trauma, which can have a profound impact on an individual's mobility and quality of life. Bone void fillers play a crucial role in the treatment of fractures, providing structural support and aiding in the healing process. The increasing prevalence of fractures, whether due to accidents, sports injuries, or age-related fragility, contributes significantly to the demand for these orthopedic solutions.
Conditions like osteoarthritis, characterized by the deterioration of joint cartilage, contribute substantially to the demand for orthopedic interventions. Bone void fillers prove valuable in these scenarios by promoting joint preservation and alleviating pain. As the prevalence of degenerative orthopedic conditions rises, so does the need for advanced solutions that can enhance joint function and improve the overall quality of life for affected individuals.
With an increasing emphasis on an active lifestyle, sports-related injuries have become more prevalent. These injuries often involve damage to bones and joints, requiring effective orthopedic interventions. Bone void fillers offer a minimally invasive option for addressing these injuries, facilitating quicker recovery times and enabling athletes to resume their activities sooner.
Advancements in surgical techniques, including bone reconstruction procedures, contribute to the growth of the bone void fillers market. Whether it's reconstructing bone defects after tumor removal or addressing congenital anomalies, bone void fillers play a pivotal role in supporting bone regeneration. The increasing adoption of these advanced surgical procedures amplifies the demand for effective and reliable bone void fillers.
Patients are increasingly inclined towards minimally invasive treatments for orthopedic issues. Traditional surgical procedures often involve prolonged recovery periods and higher risks. Bone void fillers, being minimally invasive, offer a compelling alternative, aligning with the preferences of patients who seek effective solutions with reduced postoperative complications.
Patient Preference for Non-Invasive Treatments
In the rapidly evolving landscape of healthcare, patient preferences are playing an increasingly influential role in shaping treatment choices. A notable trend contributing to this shift is the growing preference for non-invasive or minimally invasive treatments. Nowhere is this preference more evident than in the realm of orthopedic care.One of the primary reasons patients favor non-invasive treatments, such as bone void fillers, is the desire to minimize disruption to their daily lives. Traditional orthopedic surgeries often entail extended recovery periods, impacting a patient's ability to resume normal activities promptly. In contrast, non-invasive options allow for quicker recoveries, enabling patients to return to their routines sooner.
Patient apprehension about postoperative complications is a significant factor influencing treatment decisions. Non-invasive procedures, including the use of bone void fillers, are associated with lower risks and complications compared to traditional surgical interventions. This factor contributes to a sense of reassurance and confidence among patients, further fueling the demand for non-invasive orthopedic solutions.
The continuous evolution of minimally invasive technologies has expanded the repertoire of non-invasive treatment options. In the France Bone Void Fillers Market, advancements in imaging techniques, arthroscopy, and precision-guided delivery of fillers contribute to the appeal of these procedures. Patients are more inclined to choose interventions that leverage cutting-edge technologies, enhancing the overall patient experience.
Non-invasive treatments often allow for greater personalization of treatment plans. Orthopedic practitioners can tailor the use of bone void fillers to address the specific needs of each patient. This personalized approach resonates with individuals seeking treatments that are not only effective but also aligned with their unique health profiles and preferences.
The shift towards non-invasive treatments aligns with the broader trend of outpatient procedures. Bone void fillers, being minimally invasive, are conducive to outpatient settings. This convenience factor is particularly appealing to patients, as it reduces the need for prolonged hospital stays and facilitates a more streamlined treatment experience.
Favorable Reimbursement Policies
In the intricate landscape of healthcare, reimbursement policies wield considerable influence on the adoption of medical technologies. The France Bone Void Fillers Market is no exception, as favorable reimbursement policies emerge as a linchpin in driving growth and shaping the trajectory of this vital sector.Favorable reimbursement policies alleviate financial burdens for patients seeking bone void filler treatments. As these policies facilitate reimbursement for the cost of procedures and associated expenses, patients are more likely to opt for bone void fillers as a viable and accessible solution. This financial support enhances the affordability of treatments, broadening the market reach and ensuring that a diverse demographic can benefit from these orthopedic interventions.
Reimbursement policies not only benefit patients but also incentivize healthcare providers to incorporate bone void fillers into their practice. When healthcare providers are assured of reasonable reimbursement for performing these procedures, they are more likely to adopt and promote the use of bone void fillers. This, in turn, contributes to the market's growth by expanding the network of practitioners offering these innovative orthopedic solutions.
Favorable reimbursement policies create a conducive environment for research and development activities within the France Bone Void Fillers Market. Pharmaceutical companies and medical device manufacturers are more likely to invest in developing advanced fillers and associated technologies when they anticipate a supportive reimbursement landscape. This investment stimulates innovation, driving the introduction of more effective and sophisticated bone void fillers to the market.
A supportive reimbursement environment fosters competitiveness within the market. As multiple players strive to offer innovative and cost-effective solutions, patients benefit from a wider range of options. This competition not only improves the quality of available bone void fillers but also contributes to the development of products that are more efficient, safer, and aligned with the evolving needs of patients and healthcare providers.
With favorable reimbursement policies, bone void fillers can seamlessly integrate into standard treatment protocols. Healthcare providers are more likely to incorporate these interventions into their orthopedic practices when reimbursement is assured. This integration enhances the overall acceptance and adoption of bone void fillers as a mainstream orthopedic solution, positively impacting market growth.
Key Market Challenges
Regulatory Complexities
One of the primary challenges in the France Bone Void Fillers Market is navigating the intricate regulatory landscape. Stringent regulations and approval processes can pose hurdles for market players looking to introduce new products or modify existing ones. Achieving compliance with evolving regulatory standards is both time-consuming and resource-intensive, impacting the speed at which innovative bone void fillers can enter the market.Pricing Pressures
The pricing dynamics within the healthcare industry, coupled with budget constraints faced by healthcare systems, create significant challenges for the bone void fillers market. Striking a balance between offering cost-effective solutions and maintaining profitability is a delicate task. Price pressures can limit the market's potential for growth, especially in the face of increasing research and development costs associated with advanced bone void filler technologies.Potential Side Effects and Safety Concern
As with any medical intervention, concerns about potential side effects and long-term safety play a crucial role in shaping patient and healthcare provider perceptions. Addressing these concerns through comprehensive clinical trials and transparent communication is essential for establishing trust and ensuring widespread acceptance of bone void fillers. The market faces the challenge of continuously demonstrating the safety and efficacy of these interventions.Key Market Trends
Biocompatible and Bioresorbable Materials
A notable trend on the horizon is the increasing focus on biocompatible and bioresorbable materials in the development of bone void fillers. These materials, designed to integrate seamlessly with the body and be gradually absorbed over time, aim to enhance patient outcomes by minimizing the risk of adverse reactions and improving the overall biocompatibility of orthopedic interventions.Personalized Medicine Approaches
Advancements in precision medicine are permeating the field of orthopedics, and the bone void fillers market is no exception. The trend toward personalized medicine involves tailoring treatments based on individual patient characteristics, genetics, and specific orthopedic conditions. This approach not only optimizes the effectiveness of bone void fillers but also contributes to better patient outcomes and satisfaction.Innovative Imaging Technologies
The integration of innovative imaging technologies is set to transform the way bone void fillers are deployed. Advanced imaging techniques, such as 3D imaging, virtual reality, and augmented reality, are enhancing the precision and accuracy of filler placement. This trend not only improves the procedural aspect but also aids in better postoperative monitoring and assessment of treatment efficacy.Segmental Insights
Material Insights
Based on Material, Calcium Sulphate is poised to dominate the Bone Void Fillers Market in France for several compelling reasons. Firstly, its biocompatibility makes it an ideal choice for medical applications, ensuring minimal adverse reactions and promoting successful integration with surrounding bone tissues. Additionally, Calcium Sulphate exhibits excellent resorption characteristics, gradually dissolving over time as new bone formation takes place. This feature is particularly advantageous in orthopedic and dental applications, as it supports the natural healing process and eliminates the need for a secondary removal surgery. Moreover, the cost-effectiveness of Calcium Sulphate compared to alternative materials makes it an attractive option for healthcare providers seeking efficient and affordable solutions. As the demand for bone void fillers in France continues to grow, Calcium Sulphate's unique combination of biocompatibility, resorbability, and cost-effectiveness positions it as the material of choice, driving its dominance in the market.End User Insights
Based on End User, Hospitals are poised to dominate the End User segment in the France Bone Void Fillers Market for several compelling reasons. Firstly, hospitals serve as central hubs for comprehensive medical care, including orthopedic and dental procedures that often require bone void fillers. The specialized expertise available in hospitals, coupled with state-of-the-art facilities and advanced surgical capabilities, makes them the preferred choice for patients seeking bone-related treatments. Additionally, hospitals typically have established procurement processes and purchasing power, enabling them to negotiate favorable deals with suppliers of bone void fillers. The critical nature of many procedures necessitates a high level of trust in the chosen medical institution, and hospitals, with their reputation for delivering quality healthcare, are well-positioned to meet this demand. As the demand for bone void fillers in France continues to rise, hospitals, with their comprehensive medical services and procurement advantages, are poised to dominate the market's end-user segment.Regional Insights
Northern France is positioned to dominate the Bone Void Fillers Market in the country due to several strategic factors. Firstly, the region is home to a significant concentration of renowned medical institutions and research centers, fostering a hub of expertise in orthopedics and dentistry. This concentration of healthcare excellence enhances the accessibility of advanced surgical procedures, creating a higher demand for bone void fillers. Additionally, the region's strong economic infrastructure and well-established transportation networks contribute to efficient supply chain logistics, ensuring prompt and reliable access to bone void filler products. Furthermore, the proactive approach of Northern France in embracing cutting-edge medical technologies and innovations positions it at the forefront of adopting new and improved bone void filler solutions. As a result, the convergence of medical expertise, logistical advantages, and a progressive healthcare environment establishes Northern France as a dominant player in the Bone Void Fillers Market, attracting both suppliers and end-users to the region.Report Scope:
In this report, the France Bone Void Fillers Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:France Bone Void Fillers Market, By Material:
- Calcium Sulphate
- Demineralized Bone Matrix
- Tri-Calcium Phosphate
- Others
France Bone Void Fillers Market, By Form:
- Putty
- Paste
- Granules
- Gel
- Others
France Bone Void Fillers Market, By Procedure:
- Spine Fusion & Interbody Fusion
- Oral Surgeries
- Others
France Bone Void Fillers Market, By End User:
- Hospitals
- Specialty Clinics
- Others
France Bone Void Fillers Market, By Region:
- Northern France
- Southern France
- Western France
- Central France
- Eastern France
- Southwestern France
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the France Bone Void Fillers Market.Available Customizations:
France Bone Void Fillers market report with the given market data, the publisher offers customizations according to a company's specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Stryker France SASU
- Zimmer Biomet Holdings, Inc
- Smith Nephew
- Medtronic, Plc
- Johnson & Johnson Santé Beauté France
- Integra LifeSciences Services France SASU
- Arthrex France
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 72 |
| Published | November 2023 |
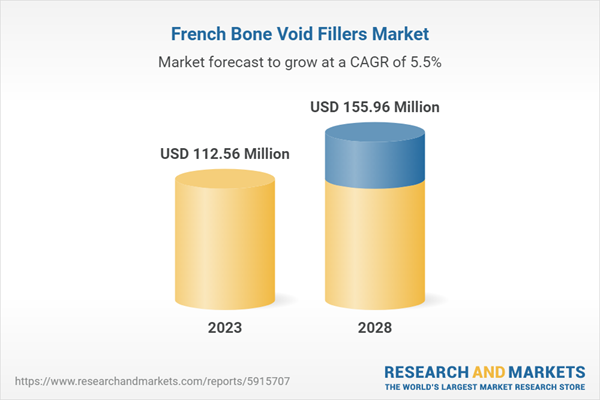
| Forecast Period | 2023 - 2028 |
| Estimated Market Value ( USD | $ 112.56 Million |
| Forecasted Market Value ( USD | $ 155.96 Million |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | France |
| No. of Companies Mentioned | 7 |