The COVID-19 pandemic had a significant impact on the neurology devices market. Due to the lockdown across the country, people were postponing their non-emergency visits for neurological diseases. As per the article from PubMed published in April 2021, it was observed that COVID-19 had a significant effect on hospital admissions and treatment for neurological diseases during 2020, and the procedures increased gradually during 2021. However, currently, the market has reached its pre-pandemic nature in terms of demand for neurology devices and is expected to witness strong growth in the coming years.
The key factors propelling the growth of the market are the increasing burden of neurological disorders, a growing number of stroke and cerebral aneurysms, and rising disposable incomes coupled with lifestyle changes.
The increasing geriatric population in the country is one of the major drivers for the market. According to the France Country Health Profile 2021 published in September 2021 by the Organization for Economic Co-operation and Development, around 20.4% of the French population was above the age of 65 years in 2021. Since the geriatric population is more prone to neurological disorders, a rise in the geriatric population is therefore anticipated to drive market growth.
Moreover, the increasing government initiatives and investments to modernize and digitize the French healthcare system are driving the growth of the market studied. In October 2021, France's Health Minister announced the investment of EUR 650.0 (USD 689.58) million to accelerate the national digital health strategy as part of the Health Innovation 2030 plan. Such initiatives may lead to the development of advanced neurological devices, which may increase the market share over the forecast period.
However, the high cost of equipment is expected to hinder market growth in the coming years.
France Neurology Devices Market Trends
Neurosurgery Devices Expected to Witness Strong Growth
Neurosurgery devices are expected to witness significant growth in the coming years. This is majorly due to the increasing number of neurological disorders in France. For instance, according to the research study published in Frontiers Organization published in September 2022, the incidence of essential tremors was considerably high in France and reported a higher value of essential tremors among people aged 60 years and older.Furthermore, PubMed data published in March 2022 stated that the rates of patients hospitalized for stroke increased among people aged under 65 years in France. Similarly, as per the report published by the Dijon Stroke Registry for France in March 2022, there will be an increase in the number of stroke cases in France by 2030 and 2050, associated with almost a doubling of healthcare costs related to stroke by then.
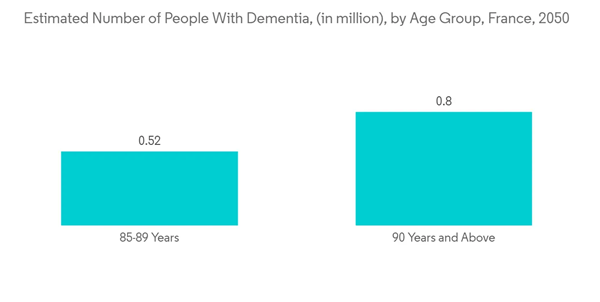
Additionally, another PubMed study published in January 2022 indicated that traumatic brain injury is a major public health concern in France, affecting mostly young patients, victims of road traffic accidents, elderly patients, and victims of falls. Intracranial pressure monitors are one of the key requirements for monitoring traumatic brain injuries. Thus, such a scenario is expected to increase the demand for the devices, thereby surging the market growth. Furthermore, the Alzheimer's Association stated that people with severe traumatic brain injury are at 4.5 times the risk of cognitive disorders such as dementia.
Interventional Neurology Devices Expected to Grow Significantly Over the Forecast Period
France's growing burden of neurological diseases is the leading factor responsible for segment growth. The French government is implementing various strategies to cope with this rising burden. For instance, in June 2021, the French Government published a Neurodegenerative Diseases Roadmap 2021-22, outlining steps that will be taken across different fields. Some of the key strategies of this roadmap include better access to research, the adaptation of hospital care (avoidance of inappropriate hospitalizations with upstream work and creation of resources for adapted hospital care), promoting French efforts in Europe, and exchanging best practices.Some companies are expanding their regional market position by adopting various strategies, such as mergers and acquisitions, while others are collaborating with companies to introduce new products to retain their market share.
For instance, in June 2020, Denali Therapeutics and its development partner, Sanofi, announced that they may stop the development of the experimental drug, DNL747, used for the treatment of Alzheimer's, and may start research on a new drug candidate DNL788 for the same indications of Alzheimer's. Sanofi is responsible for developing DNL758 and is planning clinical trials in multiple indications based on successful Phase I data. Hence, with the above-mentioned factors, the segment is believed to witness strong growth in the coming years.
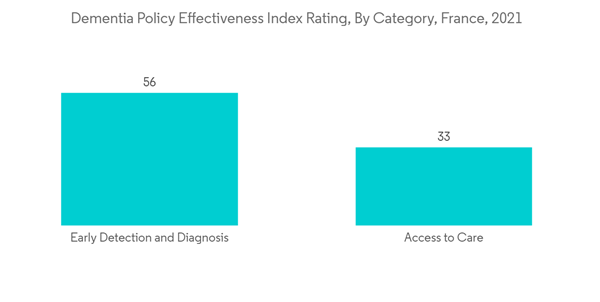
Additionally, the utilization of dementia policy is increasing in terms of awareness and monitoring purposes, fueling segment growth.
France Neurology Devices Market Competitor Analysis
France's Neurology Devices Market is moderately competitive with several major players. Some of the strategies implemented include agreements, collaborative models, business expansion, and product development. Some of the major players include B. Braun SE, Boston Scientific Corporation, Stryker Corporation, Medtronic PLC, Abbott Laboratories, Johnson & Johnson, and Smith & Nephew.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- B. Braun SE
- Boston Scientific Corporation
- Stryker Corporation
- Medtronic PLC
- Abbott Laboratories
- Johnson and Johnson
- Smith & Nephew
- Nihon Kohden Corporation