Speak directly to the analyst to clarify any post sales queries you may have.
A precise and urgent overview of clinical, diagnostic, and therapeutic complexities shaping treatment decision making and stakeholder priorities in fungal keratitis care
Fungal keratitis represents a complex clinical and commercial challenge that demands precise, multidisciplinary responses from clinicians, pharmaceutical developers, and health system planners. The condition’s heterogeneity in etiology, clinical presentation, and therapeutic response requires stakeholders to integrate microbiology, ophthalmic surgery, pharmacology, and health economics when designing care pathways. Clinicians face diagnostic constraints that influence time to effective therapy, while manufacturers and distributors must navigate formulation, delivery, and regulatory hurdles specific to ocular antifungal agents.
Against this backdrop, the treatment landscape is characterized by an interplay of topical antifungals, systemic therapies, and surgical interventions. Topical agents remain central to early management and localized infections, whereas systemic antifungal therapy is often reserved for severe or deep tissue involvement. Surgical options, including debridement and keratoplasty, are integral when medical therapy fails or when corneal integrity is threatened. Each modality imposes distinct clinical decision points and logistical requirements, and innovations in drug delivery and formulation continue to shift practice patterns.
Diagnostic accuracy and speed are pivotal determinants of outcomes and they directly affect therapeutic selection. Advances in point‑of‑care diagnostics, improved microbiological techniques, and molecular assays offer promise for earlier pathogen identification and targeted therapy. However, adoption of new diagnostics varies by care setting and resource availability, creating divergent pathways of care delivery. As a result, strategic planning for research, product development, and market access must be grounded in an appreciation of both clinical nuance and operational realities across care settings.
Emerging diagnostic precision, innovative ocular delivery systems, and supply chain resilience are reshaping therapeutic choices and commercialization pathways in fungal keratitis care
The fungal keratitis landscape is undergoing transformative shifts driven by technological advances, evolving clinical guidelines, and changing patterns of antifungal development. Novel formulation strategies, including sustained‑release topical preparations and intracorneal delivery techniques, are redefining therapeutic windows and patient adherence considerations. These innovations are supported by progressive regulatory dialogue around ocular drug delivery, which increasingly accommodates data packages tailored to localized ocular indications rather than systemic endpoints.
Concurrently, improvements in diagnostic modalities-from enhanced culture methods to molecular identification techniques-are enabling earlier and more specific pathogen-directed treatment. As a result, empirical broad‑spectrum approaches are giving way to targeted regimens when rapid diagnostics are available. This shift is complemented by greater emphasis on antimicrobial stewardship principles applied to antifungal use, encouraging optimized dosing strategies and reduced unnecessary systemic exposure.
Supply chain resilience and manufacturing quality have also moved to the forefront. Stakeholders now place higher value on robust GMP production capabilities for ophthalmic formulations and on distributed manufacturing strategies that mitigate single‑point dependencies. In parallel, payer frameworks and hospital procurement committees are increasingly scrutinizing total cost of care and comparative effectiveness, which rewards therapies and service models that demonstrably reduce surgical interventions, shorten hospital stays, or improve long‑term visual outcomes. Together, these shifts are prompting companies to align R&D priorities with deliverability, real‑world evidence generation, and integrated care models that bridge diagnostics, medical therapy, and surgical services.
Trade policy shifts and tariff pressures have spurred strategic diversification, localization of manufacturing, and contractual resilience across the antifungal supply chain
Recent tariff developments originating from trade policy changes have introduced new considerations for manufacturers, distributors, and health systems involved in the fungal keratitis supply chain. Tariff adjustments affecting raw materials, active pharmaceutical ingredients, and finished ophthalmic formulations can alter sourcing strategies and total landed costs without changing the underlying clinical value of therapies. These trade measures have prompted procurement teams and manufacturers to reassess supplier diversification, explore on‑shore or near‑shore manufacturing options, and reconfigure logistics to preserve continuity of supply.
The imposition of additional duties has also incentivized firms to evaluate vertical integration opportunities and longer term contractual structures that cushion price volatility. As a result, companies are increasingly negotiating multi‑year supply agreements and investing in regional manufacturing capacities to reduce exposure to import duties. Health systems are responding by intensifying scrutiny of vendor contracts, prioritizing suppliers with demonstrable multi‑region footprints, and exploring formulary adjustments that balance clinical efficacy with procurement stability.
Moreover, tariff pressures have accelerated conversations around local sourcing of critical inputs for antifungal formulations, particularly excipients and sterile packaging components. Regulatory authorities and procurement officials are weighing the tradeoffs between local manufacturing quality controls and cost implications, with an eye to ensuring uninterrupted patient access. Importantly, these dynamics are prompting parallel investments in logistics optimization and inventory visibility tools so that clinical teams are less likely to face supply interruptions that could compromise patient outcomes.
In sum, tariff changes have catalyzed strategic shifts across the value chain, driving supply diversification, manufacturing localization, and more rigorous procurement governance to maintain consistent access to essential antifungal therapies.
A detailed segmentation framework linking treatment modalities, drug classes, end‑user settings, and distribution channels to strategic product and commercialization decisions
Segmentation analysis reveals nuanced therapeutic and commercial pathways that influence product development and deployment strategies. Based on Treatment Modality, the market is examined across Surgical Interventions, Systemic Antifungals, and Topical Antifungals, each of which demands distinct clinical evidence, delivery logistics, and reimbursement narratives. Surgical Interventions necessitate collaboration with ophthalmic surgeons and hospital systems, Systemic Antifungals require careful consideration of systemic safety profiles and drug‑drug interactions, and Topical Antifungals must prioritize ocular tolerability and local tissue penetration.
Based on Drug Class, the landscape is organized across Azoles, Echinocandins, and Polyenes. The Azoles are further disaggregated into Imidazoles and Triazoles, with Imidazoles including Econazole and Ketoconazole and Triazoles comprising Fluconazole, Itraconazole, and Voriconazole. The Echinocandins include Caspofungin and Micafungin, while Polyenes incorporate Amphotericin B and Natamycin. These subclassifications have implications for clinical positioning, safety monitoring, and formulation choices, as each active class presents unique pharmacokinetic and pharmacodynamic profiles that influence route of administration and therapeutic sequencing.
Based on End User, the market is studied across Ambulatory Surgical Centers, Clinics, Eye Specialty Centers, and Hospitals, with Clinics further detailed into Multi‑Specialty Clinics and Specialty Clinics and Hospitals further categorized into Private Hospitals and Public Hospitals. End‑user segmentation highlights variations in diagnostic capacity, procedural throughput, and procurement practices. Ambulatory Surgical Centers and Eye Specialty Centers typically demonstrate higher specialization and faster adoption of novel surgical adjuncts, whereas public hospitals may prioritize cost containment and standardized treatment protocols.
Finally, based on Distribution Channel, the framework considers Offline Retail and Online Retail as primary pathways for product access and patient procurement. Distribution dynamics influence patient adherence, availability of compounded or proprietary formulations, and the role of specialty pharmacies. Each segmentation axis informs tailored commercialization and stakeholder engagement strategies, from clinician education programs to supply chain investments that meet the distinct needs of surgical centers, clinics, and hospital systems.
Regional clinical practice variation, regulatory diversity, and manufacturing footprints determine tailored market approaches across the Americas, Europe, Middle East & Africa, and Asia‑Pacific
Regional insights reveal differentiated clinical practices, regulatory environments, and supply chain architectures that shape access and adoption across major geographies. In the Americas, clinical networks often integrate strong tertiary ophthalmology centers with established ambulatory surgical infrastructure, enabling rapid translation of new surgical techniques and topical formulations into practice. Reimbursement and procurement pathways in this region emphasize evidence of comparative effectiveness and real‑world outcomes, which drives manufacturers to generate post‑launch data specific to diverse care settings.
In Europe, Middle East & Africa, regulatory heterogeneity and variable healthcare funding models create a mosaic of adoption patterns. Certain countries within this combined region benefit from centralized procurement mechanisms and robust hospital frameworks for corneal surgery, while others experience constraints in diagnostic capacity and access to specialized antifungal agents. Consequently, market entry strategies must accommodate differing regulatory timelines, variable pricing environments, and local clinical practice standards.
Asia‑Pacific is characterized by high clinical volume centers in urban hubs, rapidly modernizing surgical facilities, and accelerating adoption of point‑of‑care diagnostics. The region displays a strong emphasis on cost‑efficient care delivery, which drives interest in scalable topical therapies and minimally invasive surgical techniques. Moreover, manufacturing capabilities in Asia‑Pacific play a pivotal role in global supply chain planning, influencing sourcing decisions and regional partnership models. Understanding these regional nuances is essential for aligning clinical development, regulatory submissions, and commercialization strategies with the specific operational realities across the Americas, Europe, Middle East & Africa, and Asia‑Pacific.
Companies are aligning formulation innovation, diagnostic partnerships, and manufacturing resilience to demonstrate clinical value and secure preferred access within diverse care settings
Key industry participants are positioning around capabilities that bridge clinical evidence generation, formulation innovation, and supply chain robustness. Market leaders are investing in sustained‑release and targeted ocular delivery platforms to improve local drug exposure while minimizing systemic toxicity. These technology investments are frequently coupled with clinical programs that demonstrate improved tolerability and lower rates of surgical escalation, which resonate with hospital procurement and physician adoption criteria.
Companies are also strengthening diagnostic partnerships and co‑development agreements to enable companion diagnostic approaches that accelerate pathogen identification and support label‑concordant use. Strategic collaborations between formulation specialists and diagnostic firms reflect a larger trend toward integrated solutions that bundle rapid identification with therapeutic intervention. In parallel, several firms are pursuing manufacturing expansions or contract manufacturing partnerships to ensure sterility standards and reduce logistics risk for ophthalmic sterile products.
Across commercial teams, emphasis is placed on education and health economic evidence that articulates the total cost of care benefits of earlier diagnosis and effective topical therapies. Business development activity centers on geographic distribution agreements and hospital system partnerships that can secure preferred status on formularies. Finally, many organizations are building real‑world evidence platforms that capture outcomes across Ambulatory Surgical Centers, Eye Specialty Centers, and hospitals to validate product value propositions in diverse care settings and to inform iterative improvements in product design and marketing strategy.
Prioritize diagnostic‑therapeutic integration, supply chain diversification, and targeted evidence generation to accelerate adoption and protect continuity of clinical supply
Industry leaders should prioritize integrated strategies that align diagnostic acceleration with therapeutic innovation and supply chain resilience. First, invest in pathways that link rapid pathogen identification to tailored topical or systemic therapy selection, thereby reducing inappropriate broad‑spectrum use and improving clinical outcomes. A coordinated approach between diagnostics and therapeutics will also strengthen product differentiation and support favorable procurement negotiations.
Second, evaluate manufacturing footprints and sourcing strategies to mitigate tariff exposure and logistics disruptions. Near‑shore production, strategic dual‑sourcing of critical inputs, and long‑term supplier agreements will reduce vulnerability to trade policy changes and inventory shortages. Such measures should be complemented by investments in inventory visibility and demand forecasting tools to maintain consistent clinical supply.
Third, develop clinical and economic evidence that speaks directly to hospital and payer priorities. Design post‑launch studies and real‑world evidence programs that capture reductions in surgical escalation, time to clinical resolution, and overall care pathway efficiencies. Demonstrating impact on the total cost of care will be critical to securing formulary placement and institutional adoption.
Finally, tailor commercialization tactics to end‑user segmentation, engaging Ambulatory Surgical Centers and Eye Specialty Centers with surgeon‑focused clinical data while addressing procurement and standardization needs in hospitals and multi‑specialty clinics. Cross‑functional alignment between medical affairs, market access, and supply chain will ensure that product positioning, reimbursement strategies, and distribution models are coherent and responsive to local clinical realities.
A transparent, multi‑method research approach combining expert qualitative interviews, regulatory analysis, and clinical literature synthesis to validate practical market insights
This analysis synthesizes primary qualitative inputs from clinical experts, surgeons, pharmacists, and procurement leaders with secondary research drawn from peer‑reviewed literature, regulatory guidance documents, and industry technical publications. Primary engagement included structured interviews and advisory consultations to validate clinical practice patterns, diagnostic adoption barriers, and therapeutic decision‑making across different care settings. These interactions informed interpretations of clinical endpoints, safety considerations, and real‑world usage behaviors.
Secondary sources included clinical trial registries, regulatory filings, and published consensus guidelines relevant to ocular mycology and corneal infection management. In addition, manufacturing and supply chain considerations were assessed through review of manufacturing standards, sterile product guidance documents, and logistics best practices. Regional regulatory landscapes were mapped using publicly available agency directives and clinical guidance to ensure accurate representation of submission pathways and approval requirements.
Analytical approaches combined thematic synthesis of qualitative insights with structured comparative analysis across segmentation axes and regions. Triangulation between clinical expert input and documented regulatory requirements was used to refine risk assessments and to identify opportunities for localized product positioning. Throughout the methodology, emphasis was placed on transparency in source attribution and on validating assumptions through multiple independent inputs to increase the robustness and practical applicability of the findings.
Synthesize diagnostics, therapeutic innovation, and operational resilience to deliver measurable clinical benefits and secure sustainable adoption across diverse regional care systems
Fungal keratitis presents a dynamic intersection of clinical urgency, formulation science, and supply chain complexity. The path forward for stakeholders requires synchronizing diagnostic innovation, targeted therapeutic development, and operational resilience to meet clinician and patient needs. Strategic investments in ocular delivery technologies and rapid diagnostics will have outsized impact when paired with supply chain strategies that mitigate tariff and logistics risks.
Equally important is evidence generation that resonates with end‑user priorities. Demonstrating reductions in surgical intervention rates, shorter times to visual recovery, and improved tolerability will be persuasive to hospitals, surgeons, and payers. Regional strategies must be tailored to account for regulatory heterogeneity and varying procurement models across the Americas, Europe, Middle East & Africa, and Asia‑Pacific, ensuring that product introductions are both clinically aligned and operationally feasible.
In summary, stakeholders who integrate diagnostics, therapeutics, and supply chain optimization while building credible clinical and economic evidence will be best positioned to improve patient outcomes and to achieve sustained adoption in diverse care settings.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Fungal Keratitis Treatment Market
Companies Mentioned
The key companies profiled in this Fungal Keratitis Treatment market report include:- Ajanta Pharma Limited
- Alcon Inc.
- Bausch & Lomb Incorporated
- Cipla Limited
- Fresenius Kabi AG
- Gilead Sciences, Inc.
- Novartis AG
- Pfizer Inc.
- Sandoz International GmbH
- Santen Pharmaceutical Co., Ltd.
- Sun Pharmaceutical Industries Limited
- Viatris Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
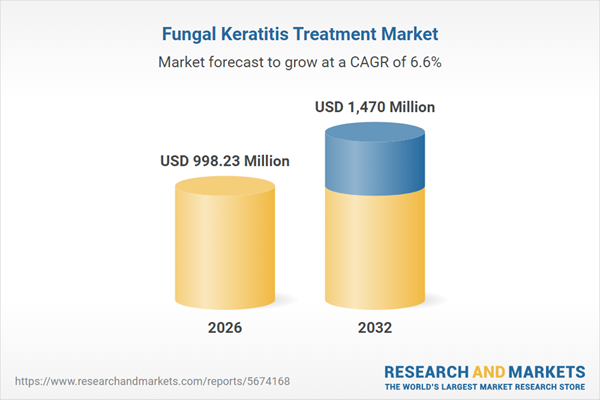
| Estimated Market Value ( USD | $ 998.23 Million |
| Forecasted Market Value ( USD | $ 1470 Million |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |