Speak directly to the analyst to clarify any post sales queries you may have.
A thorough orientation to fusion proteins that synthesizes scientific principles, translational advances, regulatory dynamics, and strategic priorities for decision makers
Fusion proteins represent a convergence of molecular engineering, translational biology, and therapeutic innovation that has reshaped modern drug development. At their core, these biologics combine functional domains from two or more proteins into a single polypeptide chain to enhance pharmacokinetics, improve target specificity, or modulate immune responses. Advances in recombinant DNA technology, precision linkers, and expression systems have moved many conceptual constructs from bench to clinic, and a growing number of regulatory approvals and late-stage clinical readouts underscore the modality's maturation.Today, fusion proteins are being developed to address complex disease mechanisms across immunology, oncology, metabolic disorders, and rare diseases. Improvements in protein design, including rational linker selection, Fc engineering for half-life extension, and modular scaffolds for multispecificity, have expanded the therapeutic toolkit while simultaneously introducing new translational challenges such as immunogenicity profiling and manufacturability at scale. As development timelines compress and competitive dynamics intensify, stakeholders must balance scientific ambition with operational discipline to maximize the probability of clinical and commercial success.
This executive summary synthesizes the prevailing scientific trends, regulatory dynamics, commercial considerations, and actionable insights that industry leaders require to navigate the fusion protein landscape. The aim is to align technical nuance with strategic decisions, providing a pragmatic framework for prioritizing investment, optimizing development pathways, and anticipating supply chain and policy headwinds that could impact program outcomes.
A landscape undergoing rapid transformation where molecular engineering, adaptive clinical strategies, regulatory refinement, and manufacturing innovation converge to reshape development and commercialization
The fusion protein landscape is undergoing transformative shifts driven by simultaneous advances in molecular engineering, clinical strategy, and commercialization pathways. Technologically, the field is moving beyond single-purpose constructs toward multifunctional molecules that combine targeting, immune modulation, and pharmacokinetic optimization in one therapeutic entity. This evolution is enabled by improved linker chemistries and recombinant DNA methods that allow designers to tune potency and half-life while mitigating aggregation and off-target effects.Clinically, developers are embracing adaptive trial designs and biomarker-driven cohorts to accelerate proof-of-concept while minimizing exposure to ineffective regimens. As a result, the translational pathway is more iterative: early clinical data informs rapid design cycles, enabling next-generation constructs to be optimized in response to real-world pharmacology. Concurrently, regulatory agencies are refining frameworks for complex biologics, placing greater emphasis on robust immunogenicity assessment, analytical comparability, and real-world evidence to support lifecycle management.
Commercially, payers and purchasers are increasingly focused on demonstrable value through real-world outcomes and durable patient benefit. This shift is catalyzing closer integration between clinical development and HEOR activities, requiring teams to design trials that capture long-term endpoints and health-economic metrics from the outset. Manufacturing innovation is also a key axis of change, with expanded use of contract development and manufacturing organizations, platform expression systems, and single-use technologies that improve flexibility and time-to-clinic. Taken together, these shifts create a landscape where technical excellence must be matched by agile clinical strategies and supply chain resilience to convert scientific potential into patient impact and commercial success.
Anticipated tariff dynamics in 2025 shaping sourcing strategies, manufacturing footprint decisions, and pricing negotiations across the fusion proteins value chain
Anticipated tariff adjustments in the United States for 2025 are expected to have material implications for fusion protein development and commercialization, with cascading effects across procurement, manufacturing location decisions, and pricing strategy. Increased duties on raw materials, disposable bioprocess components, and laboratory equipment can raise the landed cost of goods for developers that rely on international suppliers. As a consequence, many organizations will need to reassess supplier contracts, scrutinize total landed costs, and explore nearshoring or regional sourcing to mitigate exposure to import tariffs.Beyond direct procurement impacts, tariffs can influence strategic decisions regarding where to site biomanufacturing capacity. Higher import costs may incentivize investment in domestic or regional manufacturing hubs, accelerating conversations around capital allocation for new facilities or expanded capacity with local contract manufacturers. However, transitioning production footprint is neither instantaneous nor costless; lead times, regulatory transfers, and validation activities introduce complexity that companies must plan for proactively.
In parallel, tariffs can exert indirect pressure on pricing and reimbursement discussions. Payers and health systems under cost containment pressures may require clearer evidence of economic value, particularly if list prices need to adjust to offset higher production costs. To manage this dynamic, developers should enhance supply chain transparency, build scenario models that account for tariff-driven cost variability, and engage early with procurement stakeholders to communicate cost drivers and potential mitigations. In sum, the tariff environment in 2025 will likely compel a strategic rebalancing across sourcing, manufacturing, and pricing policies to preserve both access and margins.
Integrated segmentation insights revealing how product type, clinical application, end user requirements, enabling technologies, formulation choices, and distribution channels shape development and commercialization
Segmentation analysis of the fusion proteins landscape reveals nuanced opportunities and distinct development priorities when examined by Type, Application, End User, Technology, Form, and Distribution Channel. Based on Type, the field encompasses Cytokine Fusion Proteins designed to modulate immune signaling; Enzyme Fusion Proteins that supply or augment catalytic activity; Fc-Fusion Proteins engineered for extended half-life and effector functions; and Growth Factor Fusion Proteins intended to stimulate cellular repair and regeneration. Each type carries unique analytical, safety, and manufacturing considerations that influence candidate selection and downstream development choices.Based on Application, therapeutic focus areas include Autoimmune Disorders where precision immunomodulation is prized; Cardiovascular Diseases that demand potent, durable biologic activity with an emphasis on safety; Infectious Diseases where rapid onset and scalable manufacturing are critical; Metabolic Disorders where combinatorial approaches can be transformative; and Oncology, which often requires complex targeting and combination strategies. These application areas prioritize different translational milestones, regulatory endpoints, and commercial access pathways.
Based on End User, adoption patterns are driven by Diagnostic Laboratories that require compatible assay reagents and companion diagnostics; Hospitals and Clinics that focus on administration logistics and inpatient workflows; Pharmaceutical and Biotechnology Companies that prioritize scalable manufacturing and IP protection; and Research Laboratories that emphasize flexibility and early-stage innovation. Each end user group imposes distinct requirements on product format, labeling, and support services.
Based on Technology, the development landscape is shaped by Cross Linking Technology which enables stable multimers and scaffolds; Peptide Linker Technology that fine-tunes inter-domain spacing and protease sensitivity; and Recombinant DNA Technology which underpins expression, vector design, and host-cell optimization. Technology choices materially affect manufacturability, analytical strategy, and regulatory submissions. Based on Form, product presentation matters: Liquid formulations facilitate ready-to-administer dosing but can pose stability challenges, whereas Lyophilized formats extend shelf life and cold chain flexibility but require reconstitution workflows and validation. Based on Distribution Channel, commercialization strategies vary across Direct Sales models that favor close customer relationships, Distributors that expand geographic reach, and Online Channels that accelerate ordering for research-use products and selected commercial offerings. Integrating these segmentation lenses yields a pragmatic view of where scientific innovation aligns with clinical demand and commercial execution.
Regional dynamics and strategic considerations across the Americas, Europe Middle East & Africa, and Asia-Pacific that determine development priorities, manufacturing decisions, and market access approaches
Regional dynamics exert a powerful influence on clinical development pathways, manufacturing strategy, and commercialization approaches, creating differentiated priorities across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, robust venture funding ecosystems, advanced clinical trial infrastructure, and an emphasis on rapid regulatory engagement continue to attract early-stage innovation and late-stage investment. This environment favors developers who can demonstrate clinical differentiation and build strong payer evidence early in the lifecycle to support access decisions.Across Europe, Middle East & Africa, regulatory harmonization within major markets and an increasing focus on health technology assessment drive an emphasis on real-world outcomes and cost-effectiveness. Stakeholders operating in these regions must plan for multi-jurisdictional regulatory pathways and vary HTA strategies to meet diverse reimbursement criteria. The region’s manufacturing base and specialized contract manufacturers also offer strategic opportunities for companies seeking high-quality production capacity.
In Asia-Pacific, rapid market expansion is accompanied by investments in biomanufacturing capacity, a growing base of skilled talent, and regulatory agencies that are progressively aligning with international standards while adopting pragmatic pathways for innovative therapies. For many developers, Asia-Pacific presents attractive options for cost-efficient manufacturing and large patient populations for trial recruitment, but success requires nuanced market access strategies and local partnerships to navigate payer systems and distribution networks. Across all regions, companies must reconcile global development plans with local regulatory expectations, supply chain resiliency, and payer evidence requirements to ensure successful launch and sustained uptake.
Competitive landscape insights highlighting the roles of established biopharma, agile biotech innovators, CDMOs, and academic spinouts in shaping platform evolution and commercial execution
Competitive dynamics in the fusion protein sector are defined by a mixture of established biopharmaceutical firms, nimble biotechnology innovators, specialized contract development and manufacturing organizations, and academic spinouts translating platform technologies. Established players bring deep regulatory experience, broad commercial infrastructure, and scale in manufacturing and distribution, enabling them to advance late-stage assets and orchestrate global launches. In contrast, smaller biotech companies often drive platform innovation and design-first approaches, leveraging partnerships or selective out-licensing to accelerate development while managing capital efficiency.Contract development and manufacturing organizations play a pivotal role by providing scalable production, analytical development expertise, and fill-finish capabilities that lower the barrier to entry for clinical-stage programs. Academic institutions and translational research centers continue to seed novel constructs and mechanistic insights, creating a steady pipeline of early-stage opportunities that can be spun out or licensed. Collaboration models vary from joint ventures and strategic alliances to milestone-based licensing arrangements, and successful companies tend to align partnerships to fill capability gaps-whether in clinical development, regulatory affairs, or large-scale manufacturing.
Intellectual property strategy, regulatory track record, and manufacturing robustness are recurring differentiators. Firms that invest in platform robustness, analytical comparability packages, and early engagement with regulators are better positioned to navigate complex biologics pathways. Moreover, given the modality’s sensitivity to immunogenicity and stability, organizations that integrate orthogonal analytical techniques and invest in predictive immunogenicity assessments secure a competitive edge during review and post-approval lifecycle management.
Actionable strategies for leaders to align platform design, regulatory engagement, supply chain resilience, and payer-focused evidence generation to accelerate success
Industry leaders must adopt a strategic combination of technical rigor, supply chain resilience, and commercial foresight to capitalize on fusion protein opportunities. First, prioritize modular design principles that allow platform reuse across indications; investing in robust linker strategies and Fc engineering can shorten development cycles and reduce downstream comparability risk. Second, integrate health economics and outcomes research early in clinical planning to ensure trial endpoints capture payer-relevant evidence and support reimbursement conversations at launch.Third, proactively de-risk supply chains by diversifying supplier bases, exploring nearshoring of critical components, and qualifying multiple contract manufacturing partners to minimize disruption from trade policy shifts and capacity bottlenecks. Fourth, cultivate regulatory relationships and submit comprehensive analytical comparability packages; early scientific advice can reduce review uncertainty and accelerate approval timelines. Fifth, structure partnership agreements to balance capital efficiency with control over key value chain elements, using milestone-based licensing and strategic co-development where appropriate.
Finally, invest in post-market evidence generation and pharmacovigilance to demonstrate long-term value and safety. Real-world data strategies that capture durability, quality of life, and healthcare utilization will be crucial for sustaining reimbursement and expanding label indications. By combining platform robustness, commercial alignment, and operational redundancy, organizations can translate scientific promise into durable patient access and commercial returns.
Transparent and reproducible research approach combining systematic secondary review, primary expert interviews, and rigorous cross-validation to underpin conclusions and recommendations
The research methodology underpinning this analysis combines a structured review of peer-reviewed literature, regulatory filings, proprietary primary interviews, and validated industry sources to build an accurate and defensible perspective on fusion proteins. Secondary research included systematic analysis of scientific publications, clinical trial registries, regulatory guidance, and patents to map technological trends, clinical endpoints, and analytical best practices. This provided the technical foundation that informed hypothesis development and the design of primary instruments.Primary research comprised in-depth interviews with senior executives, clinical development leads, manufacturing experts, and payers to validate assumptions, surface operational challenges, and capture forward-looking priorities. Interview topics included linker design trade-offs, immunogenicity strategies, manufacturing scale-up barriers, and commercialization hurdles. All qualitative inputs were cross-validated against secondary evidence to reduce bias and ensure consistency.
Analytical rigor was applied through triangulation of sources, sensitivity testing of key assumptions, and peer review by subject-matter experts. Wherever applicable, regulatory precedents and documented comparability cases were used to ground recommendations. Limitations are acknowledged: proprietary commercial terms and confidential pipeline data were not directly accessible and thus the analysis focuses on observable trends and validated expert opinion rather than undisclosed internal metrics. The methodology is transparent, reproducible, and designed to support strategic decision making across development, manufacturing, and commercial functions.
A concise synthesis emphasizing the importance of aligning platform engineering, evidence generation, and supply chain resilience to transform fusion protein innovation into patient impact
Fusion proteins occupy a distinctive and rapidly evolving niche within biologics, offering the potential to address unmet clinical needs through engineered multifunctionality and improved pharmacologic profiles. Scientific advances in linker chemistry, Fc engineering, and recombinant expression systems have reduced several historical barriers to development, but the modality still requires careful attention to immunogenicity, stability, and scalable manufacturing. Strategic alignment between early design decisions and long-term commercialization objectives is essential to realize therapeutic and commercial value.Regulatory and payer landscapes are maturing in step with technical progress, placing a premium on robust analytical packages, real-world evidence, and clear demonstrations of clinical benefit. Operationally, the interplay between sourcing, manufacturing footprint, and trade policy requires active management to preserve margins and ensure uninterrupted supply. Looking forward, organizations that adopt platform-oriented engineering, prioritize payer-relevant endpoints, and build resilient supply chains will be best positioned to convert innovation into sustainable patient impact. The insights and recommendations provided here are intended to help stakeholders prioritize investments, refine development strategies, and engage the ecosystem in ways that accelerate safe and effective therapies to patients.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Fusion Proteins Market
Companies Mentioned
- Abcam plc
- Agilent Technologies, Inc.
- Amryt Pharma plc
- Astellas Pharma Inc.
- AstraZeneca PLC
- Bio-Techne Corporation
- Biocon Limited
- Catalent, Inc.
- Danaher Corporation
- GenScript Biotech Corporation
- Merck KGaA
- Novartis AG
- Novo Nordisk A/S
- Oramed Pharmaceuticals Inc.
- PerkinElmer, Inc.
- Pfizer, Inc.
- Proxima Concepts Limited
- Qiagen N.V.
- Takara Bio, Inc.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
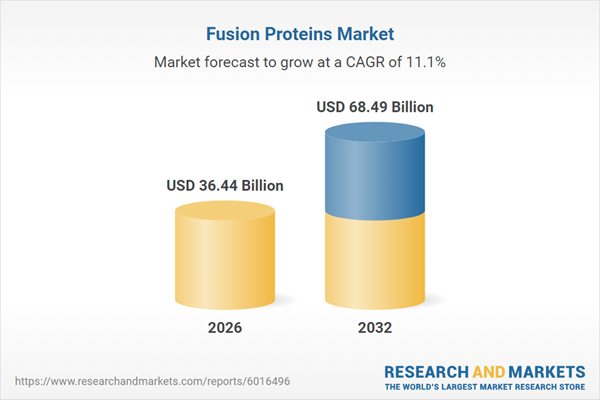
| Estimated Market Value ( USD | $ 36.44 Billion |
| Forecasted Market Value ( USD | $ 68.49 Billion |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |