Speak directly to the analyst to clarify any post sales queries you may have.
The gemifloxacin market is experiencing sustained growth, driven by evolving clinical needs, strategic regulatory oversight, and renewed focus on resilient pharmaceutical supply chains. Senior decision-makers require an integrated view of how these forces intersect to shape both near- and long-term opportunities for stakeholders across care settings and value chain segments.
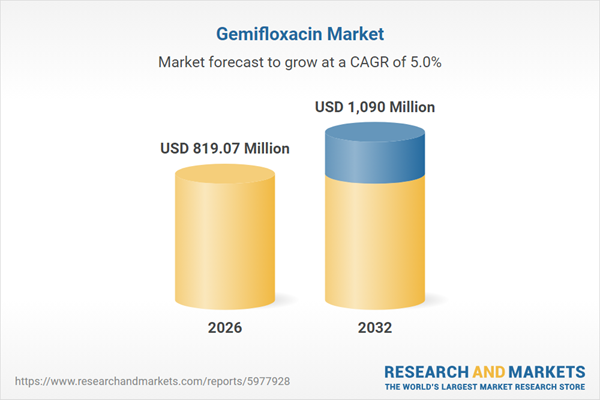
Market Snapshot: Gemifloxacin Market Size and Outlook
The Gemifloxacin Market grew from USD 777.74 million in 2025 to USD 819.07 million in 2026. It is expected to continue growing at a CAGR of 4.99%, reaching USD 1.09 billion by 2032. The market’s upward trend reflects a combination of evolving clinical utilization, adherence to updated safety guidelines, and adaptive strategies to mitigate supply disruptions and regulatory challenges. These dynamics influence formulary decisions, procurement cycles, and commercial strategy for participants operating in this sector.
Scope & Segmentation
- Clinical Indications: Focused on acute bacterial exacerbations of chronic bronchitis and community-acquired pneumonia, with utilization affected by evolving resistance data and stewardship protocols.
- Distribution Channels: Includes hospital pharmacies, online pharmacies, and retail pharmacies, each with distinct inventory strategies and regulatory requirements.
- End Users: Hospitals, ambulatory surgical centers, and clinics drive varied purchasing dynamics, care delivery models, and inventory practices.
- Regional Markets: Analysis covers the Americas, Europe, Middle East & Africa, and Asia-Pacific, where regulatory and supply chain environments present differentiated opportunities and challenges.
- Supply Chain Focus: Emphasizes sourcing diversity, manufacturing resilience, and procurement practices in response to regulatory and tariff-driven shifts.
- Technological Developments: Integration of rapid diagnostics and telehealth supports targeted prescribing and outpatient management, influencing product demand and deployment channels.
Primary Keyword: Gemifloxacin Market—Key Takeaways for Decision Makers
- Regulatory agencies apply close scrutiny to gemifloxacin, influencing approval, surveillance, and targeted education for prescribers.
- Antimicrobial stewardship programs prioritize restrictive use and ongoing risk mitigation, demanding data-driven formulary updates.
- Distribution and procurement pathways are adapting to evolving supply chain risks, promoting supplier diversification and increased transparency.
- Rapid diagnostic technologies and digital health adoption enable more refined, patient-centric utilization in both inpatient and outpatient settings.
- Segment-specific strategies, informed by end-user dynamics and distribution model, support operational efficiency and clinical effectiveness.
- Regional variation in adoption and regulatory guidance requires tailored engagement and flexible go-to-market approaches.
Tariff Impact: 2025 Policy Shifts and Supply Chain Strategies
Recent U.S. tariff changes on active pharmaceutical ingredients and packaging have increased input costs and prompted a reevaluation of supplier portfolios within the gemifloxacin market. Pharmaceutical manufacturers are responding through supplier diversification, localized production initiatives, and renegotiation of supply terms to enhance resilience. These policy developments have also elevated industry dialogue around domestic manufacturing capabilities, strategic stockpiling, and procurement risk mitigation—pushing procurement leaders to weigh supply chain continuity and total cost of ownership more heavily in decision processes.
Methodology & Data Sources
The research leverages a mixed-methods approach, combining structured interviews with clinicians, pharmacists, and supply chain executives alongside analysis of regulatory documents, peer-reviewed literature, and procurement data. Triangulation of these data streams underpins credible and actionable insights. Analytical safeguards include peer review, transparent documentation of interview protocols, and rigorous sensitivity testing of key assumptions.
Why This Report Matters
- Supports evidence-based decision-making for clinical, procurement, and commercial leaders navigating regulatory, supply chain, and operational complexity.
- Enables benchmarking of segment strategies and identification of best practices in stewardship, supply resilience, and market access.
- Delivers reliable insights rooted in the latest real-world data and regulatory trends, informing strategic planning and risk management.
Conclusion
Success in the gemifloxacin market requires balance across clinical stewardship, regulatory vigilance, and supply chain resilience. Strategic alignment of these priorities helps ensure responsible utilization and long-term market sustainability for all healthcare stakeholders.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
15. China Gemifloxacin Market
Companies Mentioned
The key companies profiled in this Gemifloxacin market report include:- Aprogen KIC Inc.
- Boryung Pharmaceutical Co. Ltd.
- Celltrion Inc.
- Chong Kun Dang Pharmaceutical Corp.
- CJ CheilJedang Corp.
- CJ HealthCare Corporation
- Daewoong Pharmaceutical Co. Ltd.
- Dong-A ST Co. Ltd.
- Dongwha Pharm Co. Ltd.
- EuBiologics Co. Ltd.
- GC Pharma
- Green Cross Corporation
- HanAll Biopharma Co. Ltd.
- Hanmi Pharmaceutical Co. Ltd.
- Huons Global Co. Ltd.
- Ildong Pharmaceutical Co. Ltd.
- Jeil Pharmaceutical Co. Ltd.
- JW Pharmaceutical Corporation
- Kunwha Pharmaceutical Co. Ltd.
- Kwang Dong Pharmaceutical Co. Ltd.
- LG Chem Ltd.
- Myungmoon Pharm Co. Ltd.
- Samjin Pharmaceutical Co. Ltd.
- Shin Poong Pharmaceutical Co. Ltd.
- Yuhan Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 819.07 Million |
| Forecasted Market Value ( USD | $ 1090 Million |
| Compound Annual Growth Rate | 4.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |