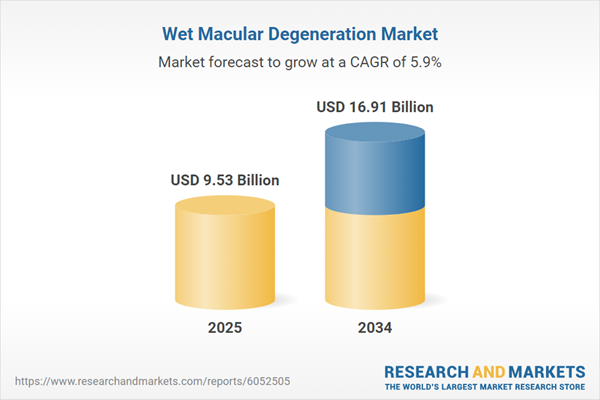
Wet Macular Degeneration Market Overview
Wet macular degeneration (or neovascular AMD) is a long-term disease that affects central vision in senior citizens. Wet macular degeneration is a more serious condition and is a type of AMD marked by a severe, rapid loss of central vision in the retina where macular degeneration has occurred. This condition develops when the small blood vessels beneath the macula of the retina swarm and ooze out fluids like blood. While the rest of the retina is involved in the peripheral vision, the macula is responsible for the central vision, necessary for reading or identifying faces. Symptoms are effectively controlled by early intervention, and medications like anti-VEGF injections can delay the progression of the condition.Wet Macular Degeneration Market Growth Drivers
Increasing Aging Population to Boost the Market Growth
In a report, the World Health Organization (WHO) highlighted that with increasing life expectancy, a large number of individuals will reach 60 or older by 2050. Wet macular degeneration cases are bound to surge with the increasing incidence of the geriatric population base, leading to increased demand for treatment among the elderly with vision-related diseases.FDA Approval Boosts the Market Growth
In September 2024, Pavblu, the fifth biosimilar referencing Eylea (aflibercept), was approved by the FDA for the treatment of retinal conditions such as wet age-related macular degeneration. Pavblu, developed by Amgen, was approved after a phase 3 study with 576 patients from various countries. It is one of several biosimilars that got approval in 2024, along with Yesafili, Opuviz, Ahzantive, and Enzeevu. There is a growing emphasis on early detection and preventive healthcare. Increasing approvals from essential regulatory authorities is expected to boost the market value in the forecast period.Wet Macular Degeneration Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Development of Long-Acting Treatments
Companies are investigating extended-duration therapies for wet AMD to decrease the need for invasive surgeries and enhance patient adherence. Innovations such as port delivery systems and sustained-release formulations are designed to prolong injection intervals, possibly decreasing treatment demands and improving patient quality of life.Emergence of Gene and Stem Cell Therapy to Boost Wet Macular Degeneration Market Demand
Gene and stem cell therapies demonstrate promise in targeting AMD. Researchers are striving to repair genetic mutations linked to wet macular degeneration and restore damaged retinal cells. Research studies show that there is a possibility of restoring vision and preventing disease progression in wet macular degeneration, indicating that these treatments may have a significant effect on the market for this condition.Adoption of Artificial Intelligence (AI) and Machine Learning (ML) in Diagnosis
Artificial Intelligence and machine learning are changing the landscape of retinal disease diagnosis by facilitating early detection and monitoring of macular degeneration, thus enhancing patient outcomes with prompt intervention. AI-driven diagnostic technology examines OCT images, recognizing levels of illness and tailoring treatment strategies.Advancements in Treatment Options Drive Market Growth
New developments in the field of ophthalmology and biotechnology have made it possible to develop ways of treating wet macular degeneration. Novel technologies such as gene therapies and new ways of drug delivery as well as heightened interest in patient-specific approaches are stimulating the development of the market.Wet Macular Degeneration Market Segmentation
The report offers a detailed analysis of the market based on the following segments:
Market Breakup by Disease Stage:
- Early-stage AMD
- Intermediate AMD
- Late-stage AMD
Market Breakup by Treatment Type:
- Drugs
- Anti-vascular Endothelial Growth Factor
- Dietary Supplements
- Others
- Devices
- Glasses
- Contact Lenses
- Others
- Surgery
Market Breakup by Route of Administration:
- Intravenous Route
- Intravitreal Route
Market Breakup by End User:
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Homecare Settings
- Others
Market Breakup by Region:
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Wet Macular Degeneration Market Share
Market Segmentation Based on the Route of Administration Set to Witness Substantial Growth
The market is segmented into intravenous route and intravitreal route based on the route of administration. Among these, the intravitreal method is expected to dominate the market because it directly administers drugs into the eye, resulting in improved treatment results and fewer overall side effects. Medications such as Ranibizumab (Lucentis) and Aflibercept (Eylea) are often given through injections directly into the eye. This technique enables focused treatment of the retina, effectively preventing the growth of abnormal blood vessels. Due to its established efficacy and lower risks when compared to systemic delivery, intravitreal therapy continues to be considered the preferred treatment method for wet AMD.Segmentation Based on End User Holds a Significant Market Share
The market is divided intohospitals, clinics, ambulatory surgical centers, homecare settings, and others. Among these, hospitals and clinics are expected to dominate the market because of their specialized ophthalmologists and advanced diagnostic equipment. They provide thorough treatment, which includes giving anti-VEGF medications such as Lucentis and Eylea via injections into the eye. Ambulatory surgical centers also hold a substantial share due to their affordability, while homecare settings are utilized for remote monitoring and occasional medical care.Wet Macular Degeneration Market Analysis by Region
Based on region, the market report covers the United States, EU-4, and the United Kingdom (Germany, France, Italy, Spain, United Kingdom), Japan, and India. Due to advanced healthcare systems and substantial investment in research, the United States is expected to dominate the market. Factors like increasing elderly populations and lifestyle-related risks impact the market in this area. The government's backing promotes market expansion through leadership in clinical trials and innovative treatments such as targeted therapies and immunotherapies. The market is growing due to greater emphasis on early detection and personalized treatment choices.Leading Players in the Wet Macular Degeneration Market
The key features of the market report include patent analysis, clinical trials analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies are:Bayer AG
Bayer AG, headquartered in Leverkusen, Germany, is a leading pharmaceutical company in the age-related wet macular degeneration (AMD) market. Their primary product, Eylea (aflibercept), is a very effective treatment for wet AMD that can preserve and even improve vision. Bayer remains committed to investing in the research and development of new AMD therapies.F-Hoffmann La Roche Ltd
Established in 1896 and headquartered in Basel, Switzerland. Roche specializes in ophthalmic disorder drugs. They developed Lucentis, a popular anti-VEGF therapy drug for wet AMD, through Genentech. They are working on new delivery methods and long-acting formulations to reduce reliance on frequent injections in AMD treatment, leveraging their biopharmaceutical expertise.Pfizer Inc
Pfizer Inc. is a significant player in the wet age-related macular degeneration (AMD) market, which is highly competitive and poised for growth due to the aging global population and the rising prevalence of eye disorders.Novartis AG
Novartis AG is a leading player in the market, primarily through its flagship drug, Lucentis (ranibizumab), a pioneering anti-VEGF therapy developed in partnership with Genentech.Other companies include Panoptica Pharma, Bausch + Lomb, Regeneron Pharmaceuticals Inc., Ocugen Inc., REGENXBIO Inc., and Oxurion NV, among others.
Key Questions Answered in the Wet Macular Degeneration Market
- What was the wet macular degeneration market value in 2024?
- What is the wet macular degeneration market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market breakup based on the disease stage?
- What is the market segmentation based on the treatment type?
- What is the market breakup based on the route of administration?
- What major factors aid the wet macular degeneration market demand?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major wet macular degeneration market trends?
- How does the rise in the geriatric population impact the market size?
- Who are the key players in the wet macular degeneration market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
- How does the management of intermediate AMD differ from late-stage AMD?
- What role do anti-VEGF drugs play in wet AMD treatment?
- Why is the intravitreal route the preferred method for wet AMD treatment?
- Why does the United States lead the wet AMD market?
- What factors drive the wet AMD market in EU-4 and the UK?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Bayer AG
- F-Hoffmann La Roche Ltd.
- Pfizer Inc.
- Novartis AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 9.53 Billion |
| Forecasted Market Value ( USD | $ 16.91 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |