Speak directly to the analyst to clarify any post sales queries you may have.
A Comprehensive Overview of WHIM Syndrome Unraveling the Complex Genetic, Clinical, and Therapeutic Landscape Driving Industry Focus
WHIM Syndrome represents a rare and complex immunodeficiency disorder characterized by warts, hypogammaglobulinemia, infections, and myelokathexis, driven by gain-of-function mutations in the CXCR4 receptor. Patients endure chronic neutropenia and lymphopenia that predispose them to severe viral, bacterial, and fungal infections beginning in early childhood. Traditionally, management has focused on supportive care through granulocyte-colony stimulating factor administration, periodic immunoglobulin replacement, and prophylactic antibiotic regimens to mitigate infectious episodes. While these measures have extended survival and improved quality of life, they do not address the underlying molecular pathology.In recent years, advances in genetic diagnostics and molecular imaging have transformed the clinical approach to WHIM Syndrome. Next-generation sequencing panels now enable precise mutation identification, allowing clinicians to stratify patients and anticipate clinical trajectories more accurately. Concurrently, the emergence of targeted CXCR4 antagonists and gene-editing strategies signals a shift toward disease-modifying interventions. This report delves into the evolving therapeutic landscape, synthesizing clinical findings, safety profiles, and real-world evidence to illuminate both the current state and future directions of WHIM Syndrome care.
Exploring Pivotal Technological and Regulatory Advances Redefining the Diagnosis, Management, and Treatment Paradigms for WHIM Syndrome
The WHIM Syndrome landscape is undergoing transformative change as precision medicine converges with regulatory incentives tailored for ultra-rare diseases. Breakthrough therapy designations and orphan drug incentives have accelerated the development of CXCR4 modulators, driving several candidates through late-stage clinical trials. Beyond small molecules, gene-editing platforms employing CRISPR-Cas9 hold promise to correct pathogenic CXCR4 mutations at their source, potentially offering a one-time curative approach.Simultaneously, diagnostic capabilities have matured through expanded access to next-generation sequencing and advanced flow cytometry assays, enabling earlier detection and genotype-phenotype correlation. This shift toward proactive monitoring is reinforced by the rise of digital health solutions, including telemedicine networks and remote patient monitoring devices, which support real-time management of neutrophil counts and infection markers. Collaboration among global research consortia, patient advocacy groups, and regulatory agencies has further harmonized trial protocols and streamlined data sharing, creating a more cohesive framework for innovation in WHIM Syndrome management.
Assessing the Anticipated Effects of United States 2025 Tariff Measures on WHIM Syndrome Treatment Accessibility Supply Chains and Cost Structures
The introduction of new United States tariff measures in 2025 presents both challenges and strategic inflection points for WHIM Syndrome stakeholders. Tariffs on specialty reagents, biologic excipients, and active pharmaceutical ingredients sourced internationally have increased procurement costs for clinical trial materials and commercial formulations. Manufacturers are now evaluating alternative supply chain models, including domestic production partnerships and vertical integration to stabilize input pricing and mitigate exposure to further trade policy shifts.These adjustments have practical implications for patient access and overall program economics. Payers are closely scrutinizing cost increases, prompting manufacturers to explore innovative contracting structures such as outcomes-based agreements and risk-sharing arrangements. Clinical trial sponsors are also reassessing site selection criteria to minimize logistical burdens, favoring regions with established biotech infrastructure. While tariffs introduce additional complexity, they are catalyzing more resilient supply chain strategies and spurring creative pricing models designed to preserve patient affordability without compromising long-term R&D investment.
Unveiling Key Market Segmentation Across Treatment Modalities Therapeutic Applications Administration Routes and End User Settings for WHIM Syndrome
Market segmentation analysis reveals distinct patterns in how WHIM Syndrome treatments are delivered, administered, and applied across care settings. The spectrum of treatments-ranging from granulocyte-colony stimulating factor therapy and immunoglobulin replacement to prophylactic antibiotic regimens-reflects a tiered approach to addressing neutropenia, hypogammaglobulinemia, and recurrent infections. Each modality carries unique safety considerations and resource requirements that inform clinical pathways and reimbursement discussions.Within administration routes, injectable therapies remain predominant, driven by the pharmacokinetic properties essential for maintaining sustained neutrophil counts. Oral formulations are emerging as patient-friendly alternatives, especially in home care environments where adherence support and remote monitoring can optimize outcomes. Therapeutic applications extend beyond infection management into autoimmune manifestations, including endocrine disorders and rheumatological syndromes, alongside hematological conditions such as leukopenia and thrombocytopenia. This diversity necessitates multidisciplinary collaboration to tailor interventions according to individual patient profiles.
Analysis by end user highlights varied adoption rates across home care services, hospital infusion centers, and specialty clinics. Home environments benefit from telehealth-enabled supervision, hospitals provide acute care capabilities for complex cases, and specialty clinics offer concentrated expertise that accelerates access to novel agents. Understanding these segmentation dynamics is critical for crafting targeted market access strategies and optimizing resource allocation.
Analyzing Regional Dynamics and Patient Access Factors Across the Americas Europe Middle East Africa and Asia Pacific for WHIM Syndrome Innovations
Regional dynamics shape the WHIM Syndrome landscape as stakeholders navigate diverse regulatory frameworks and healthcare infrastructures. In the Americas, the United States leads in clinical development and advanced care delivery, supported by comprehensive orphan drug pathways. Patient advocacy has spurred registry formation, enhancing real-world evidence generation, while Latin American markets are gradually building diagnostic capacity and establishing pilot treatment programs in urban centers.Across Europe, Middle East, and Africa, European Union member states benefit from centralized marketing authorizations and shared pricing negotiations, though access disparities persist between Western and Eastern nations. The United Kingdom and Germany have pioneered early access schemes, while emerging markets in the Middle East and North Africa grapple with supply chain bottlenecks and limited specialist training.
In the Asia-Pacific region, Japan's orphan drug legislation has fostered rapid approval of CXCR4-targeted candidates, and China's evolving rare disease guidelines are unlocking domestic R&D investments. Australia and South Korea are enhancing reimbursement frameworks to include precision therapies, whereas India and Southeast Asia focus on building diagnostic networks and pilot care centers to bridge gaps in patient identification and disease management.
Revealing Strategic Moves and Pipeline Progress of Leading BioPharmaceutical Entities Developing WHIM Syndrome Therapies and Supportive Care Solutions
Several bioPharmaceutical entities are strategically positioning themselves to lead in the WHIM Syndrome arena through differentiated pipeline assets and collaborative partnerships. A biotechnology firm specializing in CXCR4 antagonists has advanced its lead oral candidate through pivotal trials, underscoring a robust safety profile and sustained neutrophil response. That progress has attracted interest from larger pharmaceutical corporations exploring licensing arrangements to expand global development footprint.Meanwhile, a gene therapy startup has initiated preclinical studies targeting autologous hematopoietic stem cells to correct CXCR4 mutations, leveraging a novel vector platform designed for high transduction efficiency and long-term expression. Established immunology leaders are also contributing with next-generation monoclonal antibodies engineered to modulate CXCR4 signaling, reflecting a diversified approach across modalities.
Strategic alliances between clinical research organizations and academic centers are further accelerating translational research, pooling expertise in immunogenetics and rare disease trial design. These collaborations create synergistic ecosystems that streamline data sharing, optimize trial enrollment, and foster regulatory dialogue, positioning each stakeholder to capitalize on upcoming approval opportunities.
Formulating Actionable Strategic Roadmaps for Industry Leaders to Accelerate WHIM Syndrome Innovation Commercialization and Patient-Centric Solutions
Industry leaders seeking to capitalize on WHIM Syndrome opportunities should prioritize investments in targeted CXCR4 modulators and gene-based interventions, as these represent the most promising paths to disease modification. Early engagement with regulatory authorities and the incorporation of surrogate endpoints can expedite development timelines and secure breakthrough designations that signal clinical value.Building strong partnerships with patient advocacy groups is vital to enhance trial recruitment and generate patient-reported outcome data that underscore quality-of-life improvements. Manufacturers must also design innovative pricing frameworks, including outcome-based contracts, to align with payer expectations and safeguard long-term access. Digital health platforms should be integrated into care pathways to support real-time monitoring of infection markers and treatment adherence, thereby reducing hospitalization rates and improving patient satisfaction.
Strengthening supply chain resilience in response to evolving tariff policies will require diversifying sourcing strategies and exploring regional manufacturing hubs. By adopting these actionable recommendations, stakeholders can drive breakthrough research, optimize market entry, and deliver patient-centric solutions that address the full spectrum of WHIM Syndrome challenges.
Detailing the Robust Mixed Methodology Integrating Primary Expert Interviews Secondary Data Sources and Quantitative Frameworks Ensuring Research Rigor
This research leverages a rigorous mixed methodology, beginning with exhaustive secondary analysis of peer-reviewed literature, clinical trial registries, patent filings, and public regulatory documents. We synthesized molecular and pharmacological data to construct a comprehensive therapeutic framework, mapping each treatment modality to patient outcomes and safety endpoints.Complementing secondary insights, primary expert interviews with hematologists, immunologists, regulatory affairs specialists, and senior executives provided nuanced perspectives on clinical trial design, market access barriers, and emerging reimbursement models. Qualitative findings were triangulated through a structured data validation process, ensuring consistency and relevance across diverse stakeholder viewpoints.
Quantitative frameworks were applied to segment the market across treatment types, delivery methods, therapeutic applications, and care settings. Regional and tariff impact analyses employed scenario modeling and sensitivity assessments to reveal strategic inflection points. All findings underwent internal peer review and alignment with real-world evidence to maintain methodological integrity and deliver actionable, well-substantiated intelligence.
Summarizing Strategic Learnings and Fostering Forward-Looking Perspectives to Drive Ongoing Advances in WHIM Syndrome Research Clinical Care and Collaboration
The insights presented herein illuminate the critical junctures shaping the WHIM Syndrome ecosystem, from novel disease-modifying strategies to geopolitical policies influencing supply chains. The analysis underscores the transformative potential of CXCR4 antagonists and gene-editing technologies while highlighting the importance of adaptive pricing models and robust patient engagement.Regional nuances-from the United States' established orphan frameworks to Asia-Pacific's burgeoning innovation hubs-demonstrate that tailored market entry and access strategies are essential. Segmentation by treatment, administration route, therapeutic application, and care setting reveals opportunities to optimize resource allocation and enhance patient outcomes.
As stakeholders converge on precision medicine and collaborative research platforms, the recommendations offered serve as a blueprint for accelerating development and ensuring sustainable access. By leveraging these strategic learnings, industry leaders can foster a patient-centric future, drive ongoing advances in clinical care, and build a resilient pipeline that meets the complex needs of WHIM Syndrome patients worldwide.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Treatment
- Granulocyte-Colony Stimulating Factor
- Immunoglobulin Replacement Therapy
- Prophylactic Antibiotic Treatment
- Mode of Administration
- Injectable
- Oral
- Therapeutic Application
- Autoimmune Conditions
- Endocrine Disorders
- Rheumatological Syndromes
- Hematological Disorders
- Leukopenia
- Thrombocytopenia
- Infection Management
- Autoimmune Conditions
- End User
- Home Care Settings
- Hospitals
- Specialty Clinics
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Aetna Inc.
- AstraZeneca Plc
- Beijing Wantai Biolog Pha Ent Co Ltd
- Bristol-Myers Squibb Co.
- Dr. Reddy's Laboratories Ltd
- Eugia Pharma Inc.
- GlaxoSmithKline Plc (GSK)
- Horizonscan geneesmiddelen
- Incyte Corporation
- Innovate Biopharmaceuticals, Inc.
- Intercept Pharmaceuticals
- Johnson & Johnson
- Merck & Co., Inc
- Pfizer Inc.
- Roche Holding AG
- Sanofi S.A.
- Serum Institute of India Pvt. Ltd.
- Takeda Pharmaceutical Company Limited
- Walvax Biotechnology Co., Ltd.
- X4 Pharmaceuticals, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this WHIM Syndrome market report include:- Aetna Inc.
- AstraZeneca Plc
- Beijing Wantai Biolog Pha Ent Co Ltd
- Bristol-Myers Squibb Co.
- Dr. Reddy’s Laboratories Ltd
- Eugia Pharma Inc.
- GlaxoSmithKline Plc (GSK)
- Horizonscan geneesmiddelen
- Incyte Corporation
- Innovate Biopharmaceuticals, Inc.
- Intercept Pharmaceuticals
- Johnson & Johnson
- Merck & Co., Inc
- Pfizer Inc.
- Roche Holding AG
- Sanofi S.A.
- Serum Institute of India Pvt. Ltd.
- Takeda Pharmaceutical Company Limited
- Walvax Biotechnology Co., Ltd.
- X4 Pharmaceuticals, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
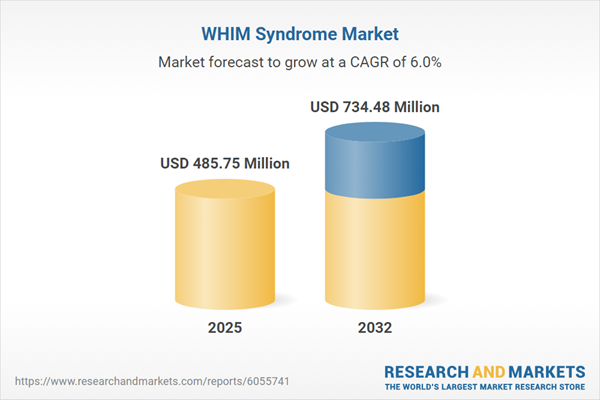
| Estimated Market Value ( USD | $ 485.75 Million |
| Forecasted Market Value ( USD | $ 734.48 Million |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |