Speak directly to the analyst to clarify any post sales queries you may have.
A strategic orientation to the evolving hemodialysis catheter ecosystem that highlights clinical drivers, device innovation, and procurement dynamics shaping care delivery
The hemodialysis catheter landscape has evolved into a technically sophisticated arena where clinical practice, device innovation, and regulatory oversight intersect to influence procurement, patient outcomes, and operational workflows. Clinicians and care providers now balance a growing emphasis on long-term catheter performance with acute access demands, while manufacturers respond with materials science improvements and tip optimization that aim to reduce complications and streamline insertion. Simultaneously, health systems are scrutinizing device selection through the lens of value-based care, seeking solutions that can demonstrably lower infection rates and minimize intervention frequency.Moreover, translational research and incremental design refinements have led to differentiated offerings across product families, with attention to biocompatibility, lumen architecture, and cuff technologies. These clinical and technological drivers are accompanied by intensified attention from regulators and payers, who increasingly require robust evidence linking device selection to measurable clinical endpoints. Consequently, procurement cycles are extending as stakeholders request comparative evidence and compatibility assessments with existing dialysis infrastructure.
In this context, stakeholders including clinicians, hospital administrators, and device developers must navigate a multi-dimensional environment where operational feasibility, patient safety, and long-term performance converge. This report synthesizes those dimensions to provide clarity on how product innovation, clinical practice shifts, and policy signals are collectively shaping near-term strategic choices across the hemodialysis catheter ecosystem.
How procedural standardization, real-world evidence demands, and supply chain resilience are converging to transform catheter selection, use, and vendor relationships
The recent period has seen transformative shifts that are redefining how hemodialysis catheters are designed, selected, and managed across care settings. Clinically, there is a clear movement toward minimizing catheter-related complications through enhanced material selection and tip design improvements, which in turn influences device preference among nephrology teams. At the same time, procedural pathways are being optimized; interventional radiology and vascular access specialists are aligning around standardized insertion techniques and imaging-assisted placement to reduce mechanical complications and improve long-term patency.Concurrently, regulatory frameworks and quality metrics have elevated the visibility of catheter performance in broader hospital quality portfolios. These shifts compel device makers to prioritize post-market surveillance and to generate real-world evidence that supports product differentiation. On the commercial side, supply chain resilience and procurement rationalization are prompting larger health systems to consolidate vendor relationships and prioritize suppliers that can demonstrate continuity of supply and responsive service models. Thus, commercial negotiations increasingly factor in logistics, training support, and bundled service offerings alongside device specifications.
Finally, digital integration and data capture at the point of care are enabling more sophisticated tracking of device outcomes, which fosters iterative improvements and supports evidence-based decision-making. Taken together, these trends are not isolated; rather, they interact to accelerate the adoption of technologies that deliver demonstrable clinical and operational benefits while reshaping competitive dynamics across the industry.
The ripple effects of mid decade tariff shifts on sourcing, manufacturing agility, and procurement negotiations that reshaped supplier footprints and cost strategies
Policy and trade actions can materially affect device sourcing, supplier strategies, and pricing structures in the medical device sector. Tariff adjustments enacted and implemented in two thousand twenty-five introduced new cost variables that have altered procurement calculus for devices and components linked to intravascular access. These shifts prompted sourcing reviews among multinational manufacturers who evaluated the viability of regional manufacturing footprints and alternative suppliers to mitigate exposure to increased tariff costs.As a direct consequence, supply chain strategies migrated from purely cost-driven models to hybrid frameworks emphasizing regional redundancy, nearshoring, and greater vertical integration where feasible. Consequently, some manufacturers accelerated supplier diversification and inventory buffering to preserve continuity of supply for high-volume cath‑eter components. Meanwhile, healthcare providers responded by intensifying negotiations with strategic suppliers and reevaluating contract terms to share tariff-driven cost impacts across the supply chain.
Importantly, the tariff environment also affected investment decisions within the sector. Device developers reweighted capital allocation toward manufacturing flexibility and automation to reduce unit-level costs in jurisdictions with lower trade barriers. In parallel, greater attention was paid to clinical value propositions that could justify price adjustments to payers and procurement committees. Ultimately, trade policy shifts acted as a catalyst for supply chain modernization and commercial renegotiations that will continue to influence strategic positioning across the hemodialysis catheter industry.
Segmentation-driven insights revealing how product type, material selection, tip configuration, insertion site, and end‑user setting jointly determine clinical and commercial priorities
Detailed segmentation analysis reveals nuanced adoption patterns and technology priorities across product types, materials, tip configurations, insertion sites, and end users. Based on product type, the market is studied across implantable, non tunneled, and tunneled, with tunneled devices examined further by cuff type and lumen count; this distinction underscores how long-term vascular access needs drive design complexity and service expectations. Based on material, the market is studied across polyurethane and silicone, reflecting trade-offs between flexibility, thrombogenicity, and durability that clinicians weigh during selection. Based on tip type, the market is studied across rigid tip and soft tip configurations, a differentiation that influences insertion technique and thrombotic profiles. Based on insertion site, the market is studied across femoral, internal jugular, and subclavian placements, with each anatomical approach carrying distinct procedural risks and patient management implications. Based on end user, the market is studied across ambulatory surgical center, dialysis center, home care, and hospital settings, highlighting the importance of setting-specific requirements such as staff training, device handling, and infection-control protocols.When these segmentation lenses are applied collectively, a clearer picture emerges: implantable and tunneled devices designed for long-term access are prioritized where chronicity and infection control are paramount, leading clinicians to favor materials and cuff designs that support secure anchoring and biocompatibility. Conversely, non tunneled options remain relevant for short-term or emergency access needs, with tip configuration and insertion site dictating procedural approach. In outpatient and home care settings, ease of handling, training resources, and maintenance requirements assume greater importance relative to features prioritized in hospital-based interventional environments. Therefore, segmentation-driven strategy enables manufacturers and purchasers to align product portfolios and contracting strategies with differentiated clinical workflows and patient populations.
Regional contours of adoption and commercialization driven by system maturity, regulatory diversity, and capacity expansion across the Americas, Europe Middle East & Africa, and Asia Pacific
Regional dynamics continue to shape adoption patterns and strategic priorities for manufacturers and care providers in distinct ways. In the Americas, established dialysis networks and integrated health systems emphasize evidence linking device choice to clinical outcomes, with procurement processes that prioritize supply continuity and vendor partnerships that offer training and outcomes support. This region's concentration of large dialysis providers also stimulates demand for standardized product lines that can be deployed across multiple sites while supporting quality reporting obligations.Across Europe, Middle East & Africa, regulatory diversity and variable infrastructure maturity create differentiated pathways to adoption. In many European markets, robust regulatory oversight and national procurement frameworks place a premium on clinical evidence and cost-effectiveness, whereas in the Middle East and Africa, access considerations and local manufacturing capacity influence sourcing decisions. As a result, manufacturers pursue region-specific strategies that balance centralized regulatory approval with localized distribution and training programs.
In the Asia-Pacific region, demographic trends and rapidly expanding dialysis capacity have driven an intensification of both acute and chronic access needs. Providers in major markets are investing in clinician training and in adoption of devices that can reduce complication rates and support growing patient volumes. Simultaneously, Asia-Pacific presents significant manufacturing and supply chain opportunities, prompting some global suppliers to localize production or form joint ventures to accelerate market penetration. Taken together, these regional contrasts underscore the need for differentiated commercial models that respond to regulatory, infrastructural, and clinical practice heterogeneity.
Competitive landscape dynamics underscoring why integrated manufacturing scale, clinical partnerships, and service capabilities determine long term commercial advantage
Competitive dynamics are characterized by a mix of established medical device firms, specialized catheter innovators, contract manufacturers, and service-oriented dialysis providers that together create a complex ecosystem. Established device firms often leverage broad distribution networks and integrated supply capabilities to support large hospital systems and national purchasers, while specialized innovators focus on niche performance improvements such as advanced tip geometries, novel cuff materials, and coatings that target infection prevention. Contract manufacturers play a critical role by enabling scale-up of new designs and flexible production without the fixed costs of full vertically integrated operations.Moreover, partnerships and co-development agreements between device developers and clinical centers have become a salient strategy for validating differentiated product claims and accelerating clinician acceptance. At the same time, service-oriented dialysis providers can influence purchasing through centralized procurement and by defining clinical criteria that suppliers must meet to become preferred vendors. In addition, academic and clinical research partnerships increasingly inform product roadmaps, offering manufacturers a pathway to generate the evidence necessary to support adoption in conservative procurement environments.
Taken together, the competitive landscape rewards organizations that combine technical innovation with demonstrable clinical value, scalable manufacturing, and responsive post-market support. Firms that align these capabilities with targeted regional strategies and robust clinician engagement are positioned to secure preferential procurement status and long-term customer relationships.
Actionable playbook for manufacturers and providers to align evidence generation, localized production, and service driven commercial models that improve clinical outcomes and resilience
Industry leaders should prioritize a sequence of actions that bridge clinical evidence generation, supply chain resilience, and customer-centric commercial models to stay competitive. First, invest in clinical partnerships and structured real-world evidence programs that can validate device performance across diverse patient populations and insertion sites, thereby reducing procurement friction and supporting value-based conversations with payers and providers. Second, develop manufacturing flexibility through regional production nodes or strategic contract manufacturing relationships to mitigate exposure to trade policy shifts and to shorten lead times for critical components.Third, enhance post-sale support by offering comprehensive training programs, procedural schools, and digital tools that facilitate consistent insertion technique and catheter maintenance; these services improve clinical outcomes and create stickiness with large buyers. Fourth, reconfigure commercial models to include bundled service offerings or outcome-linked pricing that align incentives with provider goals for infection reduction and access longevity. Fifth, prioritize modular product architectures that allow for rapid iteration of cuff types, lumen counts, and tip configurations to meet the specific needs of different end-user settings from ambulatory centers to home care.
Finally, maintain proactive regulatory surveillance and invest in post-market surveillance infrastructure to rapidly capture safety and performance data. By sequencing these initiatives and coordinating cross-functional teams, leaders can strengthen their market position while addressing the clinical, operational, and policy drivers that shape purchasing decisions.
A rigorous mixed method research design combining primary clinician engagement and secondary technical review with triangulation and expert validation to ensure robust insights
This study employed a mixed-methods approach combining primary qualitative interviews, systematic secondary research, and cross-validated data triangulation to ensure analytical rigor and practical relevance. Primary research included structured interviews with clinicians, procurement leaders, clinical researchers, and supply chain managers to capture first-hand perspectives on device performance, insertion preferences, and procurement considerations. These conversations were complemented by technical reviews of product literature, regulatory filings, and published clinical studies to contextualize design features and safety profiles.Secondary research leveraged a broad range of credible sources including peer-reviewed clinical journals, publicly available regulatory databases, hospital procurement guidelines, and technical conference proceedings. Findings from primary and secondary streams were synthesized using triangulation methods to reconcile divergent perspectives and to identify consensus patterns. Segmentation mapping populated analytic frameworks across product type, material, tip type, insertion site, and end user, enabling comparative analysis that respects clinical nuance and operational context.
Quality controls included source verification, review by domain experts, and iterative validation rounds with clinical advisors to confirm interpretive accuracy. Finally, sensitivity checks were applied to key qualitative inferences to ensure robustness in the face of regional practice variation and heterogeneous data availability. This multi-layered methodology supports confident, actionable insights for stakeholders engaged with hemodialysis catheter technologies and procurement strategies.
Synthesis of clinical, technological, and operational imperatives highlighting the strategic priorities necessary to secure durable value in hemodialysis catheter care
In conclusion, the modern hemodialysis catheter environment is defined by the interplay of clinical necessity, device innovation, and strategic procurement pressures. Design refinements focused on materials, tip morphology, and cuff engineering are reshaping clinician preferences, while regional regulatory and infrastructural differences require manufacturers to pursue differentiated commercial and manufacturing strategies. Trade policy shifts have underscored the importance of supply chain agility, prompting a move toward regional manufacturing and strategic supplier partnerships to safeguard continuity of care.Going forward, stakeholders who integrate clinical evidence generation with flexible manufacturing and customer-centered service offerings will be best positioned to meet the evolving needs of dialysis providers and patients. Collaboration between device developers and clinical centers can accelerate the evidence base needed for adoption, and enhanced post-market surveillance will provide the performance visibility that procurement committees increasingly demand. Ultimately, the path to sustained value lies in aligning product innovation with operational realities and clinician workflows, enabling safer access and more efficient care delivery across settings.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Hemodialysis Catheters Market
Companies Mentioned
The key companies profiled in this Hemodialysis Catheters market report include:- AngioDynamics, Inc.
- Argon Medical Devices, Inc.
- Asahi Kasei Corporation
- B. Braun Melsungen AG
- Baxter International Inc.
- Cook Medical LLC
- DaVita Inc.
- Dialife SA
- Fresenius Medical Care AG & Co. KGaA
- JMS Co., Ltd.
- Medtronic plc
- Merit Medical Systems, Inc.
- Nipro Corporation
- Outset Medical, Inc.
- Rockwell Medical, Inc.
- Teleflex Incorporated
- Toray Medical Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
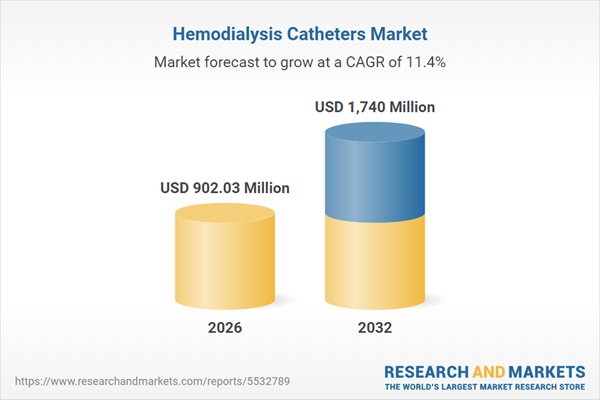
| Estimated Market Value ( USD | $ 902.03 Million |
| Forecasted Market Value ( USD | $ 1740 Million |
| Compound Annual Growth Rate | 11.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |