Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative introduction that situates clinical complexity, evolving therapeutic modalities, and health system demands within the hemoglobinopathies treatment landscape
Hemoglobinopathies encompass a group of inherited blood disorders that present persistent clinical complexity and substantial unmet medical need. Sickle cell disease and the spectrum of thalassemias produce chronic morbidity driven by hemolysis, vaso-occlusion, iron overload, and multi-organ complications, and thus demand a multifaceted response across diagnostics, acute and chronic management, and curative intent therapies. As scientific knowledge has matured, translational advances have begun to shift treatment paradigms from lifelong supportive care toward potentially curative interventions that challenge historical care models.
Consequently, clinicians, payers, and industry partners must reconcile the realities of population-level burden with evolving therapeutic modalities that range from hematopoietic stem cell transplantation to sophisticated genome-targeting approaches. Moreover, the patient journey includes complex care coordination spanning transfusion services, iron chelation, disease-modifying pharmacotherapies, and psychosocial support, which together shape real-world outcomes. Given these dynamics, a comprehensive introduction must situate clinical features alongside technological innovation, regulatory pathways, and the health-system capacities required to translate scientific promise into sustainable patient benefit.
How the convergence of gene and cell therapies, diagnostic advances, and care delivery reconfiguration is reshaping treatment and access pathways for hemoglobinopathies
The landscape of hemoglobinopathy care is undergoing transformative shifts as curative science converges with incremental improvements in supportive management. Gene therapies and advanced cell-based approaches have catalyzed a reassessment of long-term disease trajectories, prompting re-evaluation of referral patterns, transplant infrastructure, and long-term follow-up frameworks. At the same time, refinements in biologics and small molecule agents are enhancing symptomatic control and reducing acute complication rates, thereby altering quality-of-life benchmarks and clinical endpoints used in trials.
Parallel changes in health policy and payer expectations are influencing access pathways for novel therapies, while diagnostic improvements and newborn screening expansion are enabling earlier intervention. These transitions are not purely scientific; they require reconfigured care delivery networks, workforce training in cell and gene therapy logistics, and scalable models for chronic disease management. Taken together, the combined momentum of therapeutic innovation and system-level adaptation defines a period of substantive transformation with implications for investment prioritization, clinical trial design, and long-term patient outcomes.
Understanding how tariff policy and trade friction can reshape supply chains, pricing dynamics, and access to advanced therapies in hemoglobinopathy care
Tariff actions and trade measures applied to biopharmaceutical inputs, medical devices, and ancillary supplies have a ripple effect across the ecosystem that supports hemoglobinopathy care. When tariffs increase the cost base for manufacturing reagents, delivery devices, or imported therapeutics, supply chain managers and procurement teams must reassess sourcing strategies, inventory practices, and supplier relationships to maintain continuity of care. In the context of advanced therapies that rely on specialized manufacturing and cold chain logistics, incremental tariff-induced costs can complicate contract negotiations and reimbursement dialogues, even when clinical benefit is high.
Moreover, tariffs influence the strategic calculus of multinational developers who must evaluate regional production footprints, intellectual property transfer arrangements, and partner selection. As a result, some sponsors may accelerate localization of manufacturing or enter collaborative agreements with regional contract development and manufacturing organizations to mitigate exposure to trade barriers. Clinicians and health systems may experience downstream effects through pricing pressures, delayed introductions of novel products, or altered access strategies that prioritize locally sourced options. Ultimately, an appreciation of tariff impacts reinforces the importance of resilient supply chains, diversified sourcing, and proactive payer engagement to safeguard therapeutic availability for patients.
Comprehensive segmentation insight that aligns disease-specific needs, therapeutic modalities, product types, care settings, delivery routes, and age cohorts to inform strategy
Segmentation-based insight provides a structured way to interpret clinical needs and commercial pathways across disease, treatment, product, end user, route of administration, and patient age. By disease type, the field must differentiate the pathophysiology and clinical priorities of sickle cell disease versus thalassemia, since each disease group demands distinct diagnostic monitoring, transfusion strategies, and curative options. When viewed through the lens of treatment type, the divide between curative therapies and supportive care highlights a portfolio balance between high-cost, potentially one-time interventions such as bone marrow transplantation and next-generation gene therapies, and chronic supportive measures including blood transfusion, hydroxyurea therapy, and iron chelation that sustain patient stability.
Product-type segmentation clarifies that biologics and small molecule drugs follow different development and commercialization pathways; biologics such as erythropoiesis-stimulating agents and monoclonal antibodies require complex manufacturing and regulatory considerations, while small molecule approaches like hydroxyurea and L-glutamine emphasize oral delivery, adherence, and safety monitoring. End-user segmentation underscores the diversity of care settings ranging from home-based management to tertiary hospitals, research institutes, and specialty clinics, each with unique capacity to deliver transfusion services, advanced therapies, and follow-up care. Route of administration distinctions between injectable and oral therapies inform patient preference, adherence challenges, and infrastructure needs, and finally, patient age group segmentation into adult and pediatric cohorts demands age-appropriate dosing, safety surveillance, and supportive services. Together these segmentation layers enable targeted product development, differentiated pricing strategies, and tailored care pathways that align clinical benefit with operational feasibility.
How regional regulatory diversity, health system capacity, and manufacturing trends across the Americas, Europe Middle East & Africa, and Asia-Pacific shape access and deployment strategies
Regional context exerts a strong influence on clinical pathways, regulatory navigation, and commercial strategies across the Americas, Europe, Middle East & Africa, and Asia-Pacific, each presenting distinct opportunities and constraints. In the Americas, systems-level initiatives emphasize integrated care networks, genetic screening expansion, and an emerging focus on curative options that require coordination between specialty centers and payers. Meanwhile, in Europe, Middle East & Africa, regulatory heterogeneity and variable access infrastructures create a mosaic where adoption depends on local reimbursement decisions, clinical expertise in transplantation and cell therapy, and investments in diagnostic capacity.
Turning to Asia-Pacific, rapid advances in manufacturing capability, growing clinical trial activity, and expanding newborn screening programs are enabling faster translation of novel therapeutics in some markets, though disparities in health system readiness persist across the region. Cross-region differences in supply chain logistics, cold chain capacity, and healthcare workforce training are consequential for the deployment of complex biologics and gene therapies. Therefore, companies and health systems must develop regionally nuanced strategies that account for regulatory pathways, clinical infrastructure, and payer priorities to optimize patient access and long-term program sustainability.
Insights into the diverse competitive ecosystem where major pharmaceutical players, pioneering biotechs, manufacturing partners, and clinical centers converge to enable innovation
The competitive landscape in hemoglobinopathies is defined by a mix of global pharmaceutical firms, specialized biotech innovators, clinical centers of excellence, and contract service providers focused on cell and gene therapy enablement. Large pharmaceutical companies bring commercial scale, regulatory experience, and global distribution channels that can accelerate broad access once clinical and payer milestones are met. Conversely, smaller biotech companies and academic spin-outs are often the originators of disruptive modalities, particularly in gene addition and gene editing domains, and they play a pivotal role in advancing early-stage science through investigator-led trials and translational collaborations.
Complementing these developers are specialized contract development and manufacturing organizations, diagnostics firms, and clinical research networks that provide essential operational capabilities for complex therapy delivery. Health systems and specialty clinics that cultivate multidisciplinary expertise in transplantation, transfusion medicine, and long-term follow-up become de facto partners in clinical development and real-world evidence generation. Taken together, the interplay of established industry players, nimble biotech innovators, and operational enablers creates an ecosystem where strategic alliances, licensing arrangements, and co-development agreements are central to bringing new interventions from bench to bedside.
Actionable strategic recommendations that connect supply chain resilience, collaborative evidence generation, screening expansion, and capacity building to accelerate patient access
Industry leaders must adopt action-oriented strategies that bridge scientific advancement with pragmatic delivery. First, organizations should prioritize investments in supply chain resilience and regional manufacturing partnerships to reduce exposure to trade and tariff disruptions and to accelerate product availability. Second, cross-sector collaborations between developers, payers, and clinical networks should be established early to align evidence generation with reimbursement expectations, particularly for one-time curative interventions where long-term value propositions are critical. Third, expanding diagnostic and newborn screening programs in coordination with public health stakeholders can ensure timely identification and referral, which is essential for curative options to achieve their potential.
In parallel, stakeholders should invest in workforce training and center-of-excellence development to build capacity for transplantation and gene therapy administration, including long-term follow-up infrastructure for safety monitoring. Additionally, product strategies must account for patient-centric delivery models that minimize burden-favoring oral or home-based support where clinically appropriate-and must incorporate adherence support and digital health tools. Finally, companies should pursue adaptive commercialization plans that allow iterative pricing and access mechanisms tied to real-world outcomes, thereby balancing innovation incentives with equitable patient access.
A rigorous mixed-methods research approach combining expert engagement, systematic literature synthesis, pathway analysis, and validation to underpin strategic insights
The research methodology underpinning this analysis integrates multiple qualitative and quantitative approaches to ensure robustness and relevance. Primary research included in-depth interviews with clinical experts, payers, patient advocates, and operational leaders in transplantation and cell therapy centers to capture frontline perspectives on unmet needs, access barriers, and care pathways. Secondary research synthesized peer-reviewed clinical literature, regulatory guidance, and treatment guidelines to establish a common evidence baseline, while systematic review processes were applied to ensure that clinical efficacy, safety, and long-term outcomes were considered in context.
Analytical techniques incorporated comparative regulatory pathway analysis, value framework mapping, and supply chain risk assessment to understand barriers to adoption and implementation. Scenario planning and sensitivity analysis were used to evaluate implications of policy shifts, manufacturing localization, and changes in clinical practice. Finally, validation workshops with multidisciplinary experts were conducted to test assumptions, refine segmentation logic, and confirm the practical relevance of recommendations. This mixed-methods approach provides a transparent foundation for interpreting trends, prioritizing strategic actions, and supporting stakeholder decision-making.
Concluding perspective that emphasizes operational readiness, collaborative value frameworks, and patient-centered strategies to realize therapeutic advances in hemoglobinopathies
In conclusion, the hemoglobinopathy landscape is at an inflection point where scientific breakthroughs are meeting the pragmatic realities of health system delivery. The progression from supportive care models toward potentially curative interventions is reconfiguring clinical pathways, payer dialogues, and operational requirements, even as sustained improvements in biologics and small molecule therapies continue to enhance patient outcomes. These concurrent dynamics create both opportunity and complexity: opportunities to deliver transformative care and complexity in aligning manufacturing, regulatory, and reimbursement systems to support equitable access.
Therefore, stakeholders that proactively address supply chain resilience, regional regulatory variability, and capacity building stand to translate scientific promise into durable health outcomes. Equally important is the commitment to integrate patient-centered approaches, robust evidence generation, and collaborative value frameworks to ensure that novel therapies can be delivered responsibly and sustainably. In this evolving environment, strategic foresight and operational readiness will determine whether advances in gene, cell, and supportive therapies result in meaningful improvement for patients living with hemoglobinopathies.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Hemoglobinopathies Market
Companies Mentioned
The key companies profiled in this Hemoglobinopathies market report include:- Abbott Laboratories
- Alnylam Pharmaceuticals, Inc.
- Bio-Rad Laboratories Inc.
- Biogen Inc.
- Bluebird Bio, Inc.
- Bristol Myers Squibb
- CRISPR Therapeutics AG
- Danaher Corporation
- Emmaus Life Sciences Inc.
- Gamida Cell Ltd.
- Genetix Biotech Asia Pvt. Ltd
- Laboratory Corporation
- Medunik USA Inc.
- Merck & Co. Inc.
- Nexcelom Bioscience LLC
- Novartis AG
- PerkinElmer Inc.
- Pfizer, Inc.
- Prolong Pharmaceuticals, LLC
- Regenacy Pharmaceuticals, Inc.
- Sangamo Therapeutics, Inc.
- Sanofi S.A.
- Sebia
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd
- Sysmex Corporation
- Vertex Pharmaceuticals Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
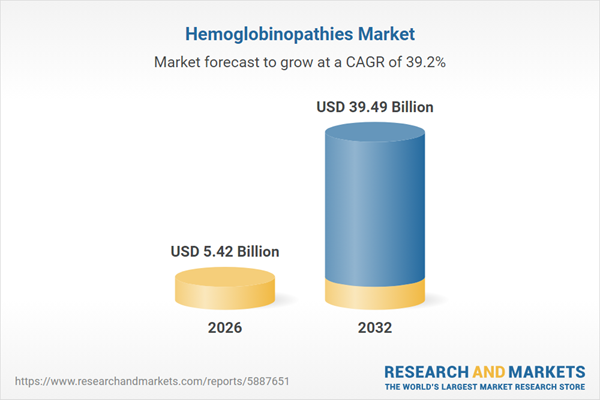
| Estimated Market Value ( USD | $ 5.42 Billion |
| Forecasted Market Value ( USD | $ 39.49 Billion |
| Compound Annual Growth Rate | 39.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |