Speak directly to the analyst to clarify any post sales queries you may have.
A strategic introduction framing clinical importance, technological convergence, and operational considerations that define the implantable cardiac monitor landscape
Implantable cardiac monitors have transitioned from niche diagnostic tools to central components of modern arrhythmia management pathways, and this introduction frames their clinical, technological, and operational relevance across contemporary cardiovascular care. The devices, ranging from minimally invasive insertables to advanced patch and band-based wearables, now sit at the intersection of continuous physiological monitoring, data-driven diagnostics, and remote patient management. Their role extends beyond episodic detection to ongoing risk stratification, therapy optimization, and longitudinal outcome measurement, thereby influencing care pathways across outpatient clinics, ambulatory surgical centers, and tertiary hospitals.Clinicians increasingly rely on longitudinal electrocardiographic data to characterize intermittent arrhythmias, guide anticoagulation decisions in atrial fibrillation, and evaluate unexplained syncope. Consequently, manufacturers and health systems are converging on integrated solutions that combine device hardware with robust software analytics, cloud-enabled data flows, and secure patient engagement tools. Technological advances-such as enhanced sensing algorithms, Bluetooth and cellular telemetry, and lower-profile form factors-are reducing barriers to sustained monitoring and expanding candidate populations.
Operational considerations are equally important. Adoption patterns are influenced by procedural settings, clinician workflow integration, reimbursement mechanisms that recognize remote monitoring value, and the availability of clinical evidence demonstrating improved diagnostic yield and downstream care benefits. Building on these foundations, stakeholders must assess device performance, interoperability, and the broader ecosystem of services that drive clinician acceptance and patient adherence. This introduction sets the stage for a deeper exploration of how clinical utility, technological maturity, and delivery models coalesce to define strategic priorities for industry and health system leaders.
Identification and analysis of the major technological, clinical, and commercial shifts that are fundamentally reshaping the implantable cardiac monitor ecosystem
The landscape for implantable cardiac monitors is being reshaped by a set of transformative shifts that span technology, care delivery, reimbursement, and strategic partnerships. First, miniaturization and battery efficiency have broadened candidacy by enabling less invasive insertion techniques and longer monitoring intervals. As a result, patient tolerance improves and the procedural burden shifts away from inpatient suites toward outpatient-appropriate settings. At the same time, sensing fidelity and noise rejection algorithms have advanced, increasing diagnostic confidence for intermittent arrhythmias and challenging prior assumptions about the relative sensitivity of insertable versus wearable approaches.Second, the integration of advanced analytics, machine learning, and cloud-native software has altered the value equation. These capabilities enable earlier signal detection, automated event triage, and the potential for predictive insights that inform preventive interventions. Interoperability standards and secure telemetry-via Bluetooth, cellular, and cloud-based architectures-are facilitating real-time clinician notifications and seamless incorporation into electronic health records, thereby streamlining clinical workflows.
Third, care pathway innovation is accelerating, with more procedures and monitoring services migrating to ambulatory surgical centers and clinic-based settings. This redistribution is supported by procedural simplification, reimbursement code evolution, and a growing preference for decentralized care delivery. Concurrently, partnerships between device manufacturers, software vendors, and health systems are becoming more strategic; companies are offering service-based models that include monitoring, data management, and clinician support rather than selling standalone hardware.
Finally, regulatory and cybersecurity imperatives are shaping product roadmaps and market entry strategies. Regulators expect robust clinical validation and post-market vigilance, while health systems demand rigorous cybersecurity protections and data governance. Together, these shifts require integrated responses that combine clinical evidence generation, technical excellence, and commercial models aligned with evolving provider needs.
A nuanced assessment of how United States tariff adjustments in 2025 have cumulatively influenced supply chains, procurement practices, and strategic sourcing decisions
Tariff adjustments announced in the United States during 2025 have exerted a cumulative influence on supply chains, procurement dynamics, and strategic planning across the implantable cardiac monitor ecosystem. The immediate effect has been to elevate the cost basis for components and finished devices when duties apply, prompting procurement teams and manufacturers to reassess supplier contracts and total landed costs. In response, many organizations have prioritized supplier diversification to reduce exposure to import duties and to mitigate single-source vulnerabilities that can interrupt device availability.Furthermore, tariff-driven cost pressures have accelerated interest in near-shoring and regional manufacturing footprints. Establishing assembly or subassembly operations closer to major end markets not only reduces tariff exposure but can shorten lead times and improve responsiveness to clinical demand. However, these transitions require capital investment and create operational complexity during the changeover period, with implications for capacity planning and quality control.
Procurement behavior has also adapted: hospitals and group purchasing organizations are placing greater emphasis on long-term contracts, total-cost-of-ownership analyses, and value-based purchasing arrangements that absorb some tariff volatility. For manufacturers, the tariffs have influenced pricing strategies, commercial terms, and decisions around contract manufacturing versus in-house production. Importantly, the cumulative impact extends beyond direct device costs to ancillary supply lines-such as batteries, electronic modules, and telemetry chips-where heightened tariffs can cascade through the value chain and compress margins.
Finally, regulatory and reimbursement considerations intersect with tariff effects. Payers evaluating cost-effective monitoring solutions may prioritize devices and service bundles with stable supply profiles and predictable pricing. As a result, tariffs have become a strategic variable that shapes long-term product planning, channel strategies, and capital allocation decisions across the sector.
Actionable segmentation intelligence connecting product types, end-user settings, clinical indications, enabling technologies, and distribution pathways to strategic priorities
Segmentation analysis offers a structured lens to translate varied product, end-user, indication, technology, and distribution attributes into practical insights for commercialization and product development. From a product perspective, the market spans devices that are designed for subcutaneous insertion and those optimized for wearable applications. Insertable cardiac monitors are differentiated by anatomical placement and implantation approach, including subcutaneous and submuscular variants that address clinician preferences for concealment, comfort, and signal stability. Wearable monitors are similarly bifurcated into band-based and patch-based form factors; bands prioritize continuous adherence over longer durations while patches emphasize single-application simplicity and patient-friendly deployment.End-user segmentation highlights where value is delivered and where clinical workflows influence device selection. Ambulatory surgical centers are increasingly attractive for implantation procedures because of streamlined workflows and lower facility costs. Clinics, including both cardiology specialty clinics and general clinics, act as key touchpoints for initial device selection, follow-up, and remote data review, while hospitals-ranging from community facilities to tertiary care centers-provide higher-acuity diagnostics and implantation settings for complex patients.
Indication-driven differentiation clarifies clinical positioning. Monitoring for arrhythmias requires devices tuned for both supraventricular and ventricular rhythm detection, whereas atrial fibrillation detection strategies must discern paroxysmal from persistent disease to guide stroke-prevention pathways. Syncope management places emphasis on episodic correlation of symptoms with rhythm disturbances and must accommodate distinctions between cardiac syncope and vasovagal mechanisms.
Technology segmentation underpins connectivity and analytics choices, spanning Bluetooth-enabled platforms with BLE or classic Bluetooth options, cloud-native solutions that use private or public cloud architectures, and wireless transport layers that depend on cellular or non-cellular protocols. These technical choices influence interoperability, latency, and data security.
Finally, distribution channel structures shape commercial access. Direct sales models, whether via manufacturer or OEM direct channels, support tight clinical training and services-based offerings. Third-party distribution-through authorized distributors or digital retailers-extends reach and can accelerate adoption among decentralized providers. Understanding how these layers interact is essential for tailoring product features, clinical claims, and commercial models that align with customer preferences and operational realities.
Comprehensive regional intelligence highlighting how differentiated adoption drivers, regulatory frameworks, and infrastructure shape strategy in major global markets
Regional dynamics exert profound influence on adoption, regulatory timing, and service models, and a nuanced regional perspective is essential for prioritizing investment and go-to-market execution. In the Americas, robust clinical networks, a mature reimbursement environment for remote monitoring, and a high prevalence of integrated electronic health record systems create fertile ground for advanced implantable monitoring solutions. The region’s procurement behaviors emphasize clinical evidence and proven workflow integration, which favors vendors able to demonstrate real-world performance and streamlined clinician support.The Europe, Middle East & Africa region presents a heterogeneous landscape in which regulatory harmonization in parts of Europe coexists with varied reimbursement frameworks and infrastructure maturity across the Middle East and Africa. In many European health systems, centralized procurement and value-based purchasing criteria prioritize long-term outcome data and total cost of care. Conversely, some markets in the Middle East and Africa are characterized by rapid private-market adoption where novel service models and hospital-led innovation can accelerate uptake, provided regulatory and distribution pathways are clear.
Asia-Pacific exhibits diverse adoption curves driven by differing healthcare budgets, manufacturing capacity, and digital infrastructure. Several markets in the region have significant device manufacturing ecosystems and are attractive for component sourcing, while others prioritize rapid clinical adoption of remote monitoring enabled by strong cellular networks and mobile-first patient engagement habits. Across Asia-Pacific, regulatory pathways and reimbursement schemes can vary widely, requiring localized evidence generation and commercial models adapted to public and private payer mixes.
Taken together, these regional distinctions influence clinical trial design, deployment sequencing, and strategic partnerships. Manufacturers and health systems must align regulatory strategy, evidence generation, and distribution models with local payer expectations and operational realities to achieve sustainable adoption across these diverse geographies.
Integrated corporate intelligence describing how product innovation, partnerships, scale, and evidence generation define competitive advantage in the implantable cardiac monitor sector
Competitive dynamics in the implantable cardiac monitor sector reflect a blend of hardware innovation, software-enabled services, and evolving business models. Leading companies are pursuing a mix of organic product development, targeted acquisitions, and strategic partnerships to expand diagnostic capabilities and to integrate monitoring services into longitudinal care pathways. The emphasis on software and analytics is particularly pronounced; firms that can combine robust sensing hardware with advanced signal-processing algorithms and clinical decision support tools are positioned to offer higher-value propositions to clinicians and payers.Partnership behavior has shifted toward ecosystem plays where device vendors collaborate with telehealth platforms, electronic health record providers, and specialist service organizations to deliver end-to-end monitoring solutions. Such partnerships reduce friction for clinical adoption and increase the stickiness of installed bases through bundled service offerings. At the same time, a subset of companies is investing in scalable, subscription-based business models that monetize ongoing monitoring, alert management, and data interpretation services rather than singular device sales.
Scale advantage remains important, especially in manufacturing, regulatory compliance, and global distribution networks. Companies with diversified production footprints can better respond to tariff-induced cost pressures and supply-chain disruptions. Intellectual property portfolios that protect sensing algorithms, miniaturization techniques, and telemetry methods are strategic assets that enable sustainable differentiation.
Finally, clinical evidence generation and post-market surveillance are central competitive levers. Organizations that prioritize robust clinical studies, real-world data collection, and outcomes research will find it easier to secure reimbursement, integrate into clinical pathways, and demonstrate comparative value to health systems and clinicians.
Practical strategic recommendations designed to help device makers, health systems, and investors convert insights into prioritized actions that accelerate adoption and mitigate risk
Industry leaders seeking to translate market intelligence into competitive advantage should pursue a coherent set of strategic actions that align product innovation with clinical workflows, supply-chain resilience, and payer priorities. First, prioritize product designs that increase procedural simplicity and patient comfort while maintaining high-fidelity sensing; these characteristics enable a shift toward outpatient and clinic-based implantation, which in turn reduces systemic friction and expands uptake. Concurrently, integrate analytics and clinician-facing decision support to reduce false positives and to deliver concise, actionable reports that fit within existing care pathways.Second, strengthen supply-chain strategies by diversifying component sourcing, evaluating near-shore manufacturing options, and negotiating flexible commercial terms that can absorb tariff volatility. Establishing contingency plans and strategic inventory buffers for critical components will reduce the risk of service interruptions during periods of geopolitical or trade-related uncertainty.
Third, invest in clinical evidence and health economic studies that demonstrate the clinical and operational benefits of continuous monitoring for key indications such as atrial fibrillation detection and syncope evaluation. These studies should be designed to answer payer-relevant questions about downstream cost avoidance and patient outcomes to support reimbursement conversations.
Fourth, build partnerships with ambulatory surgical centers, cardiology clinics, and hospital networks to pilot integrated care models that include device implantation, remote monitoring services, and clinician training. Such collaborations will accelerate adoption and provide real-world data that refine product claims.
Finally, embed cybersecurity and data governance into product roadmaps from day one. Compliance with regulatory expectations and demonstrable protections for patient data are non-negotiable prerequisites for large-scale deployment and institutional procurement.
Transparent explanation of the multi-method research approach, validation processes, and limitations that underpin the report’s insights while ensuring reproducibility
This report’s findings derive from a multi-method research approach combining primary qualitative engagements, systematic secondary intelligence review, and rigorous analytical synthesis. Primary research included structured interviews with clinicians, device engineers, procurement leaders, and payer representatives to validate clinical utility, workflow implications, and purchasing drivers. Secondary intelligence comprised peer-reviewed clinical literature, regulatory communications, product labeling, and public disclosures, all synthesized to establish a foundation for triangulation.Data validation employed cross-source corroboration and stakeholder vetting to reconcile divergent perspectives and to ensure internal consistency. The analytical framework applied a segmentation lens across product types, end users, indications, technologies, and distribution channels, enabling targeted insights and scenario-based thinking. For regional analysis, country-level regulatory and reimbursement contexts were evaluated alongside infrastructure considerations to identify practical go-to-market implications.
Where possible, clinical claims and device performance characteristics were traced to primary studies or regulatory summaries. Limitations include variability in publicly available post-market data, regional differences in reporting standards, and the rapidly evolving nature of software-enabled features that may change through incremental updates. To mitigate these limitations, the methodology emphasizes transparent assumptions, conservative interpretation of unpublished claims, and recommended follow-up through targeted primary studies.
Ethical considerations guided the treatment of stakeholder input, with anonymized quotations and aggregate synthesis used to protect proprietary perspectives. This methodology supports reproducible insights while acknowledging the need for ongoing evidence collection as technologies and policies continue to evolve.
A concise synthesis tying clinical importance, strategic priorities, and operational imperatives together to guide executive decision-making and investment focus
The concluding synthesis reaffirms the clinical and strategic importance of implantable cardiac monitors as enablers of continuous cardiac surveillance, improved diagnostic yield, and more connected care pathways. Technological advances-encompassing miniaturized hardware, enhanced telemetry, and cloud-native analytics-are not only expanding clinical indications but also reshaping commercial models toward service-oriented offerings. These dynamics create distinct imperatives for manufacturers and health systems: prioritize clinical evidence, ensure operational compatibility, and design products and services that reduce clinician burden while enhancing diagnostic confidence.Macroeconomic and policy developments, such as tariff adjustments and changes in reimbursement paradigms, impose additional strategic considerations. Organizations that proactively address supply-chain resilience and align commercial terms with payer expectations will be better positioned to sustain adoption momentum. Equally important is the regional tailoring of strategies; regulatory complexity and payer heterogeneity require localized evidence generation and distribution models.
Ultimately, success in this market depends on a balanced focus: technical excellence in sensing and connectivity; robust software and analytics that deliver clinician-relevant insights; strong clinical validation that addresses outcomes and value; and resilient commercial and operational models that withstand external shocks. Executives who integrate these elements into a cohesive strategy will be well placed to capture long-term clinical and commercial value as continuous cardiac monitoring becomes a standard component of arrhythmia management and preventive cardiology.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Implantable Cardiac Monitor Market
Companies Mentioned
The key companies profiled in this Implantable Cardiac Monitor market report include:- Abbott Laboratories
- Angel Medical Systems, Inc.
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Cook Group Incorporated
- Edwards Lifesciences Corporation
- GE HealthCare Technologies Inc.
- Integer Holdings Corporation
- Koninklijke Philips N.V.
- Lepu Medical Technology (Beijing) Co., Ltd.
- Medtronic plc
- MicroPort Scientific Corporation
- Nihon Kohden Corporation
- Shree Pacetronix Ltd.
- Siemens Healthineers AG
- Zoll Medical Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
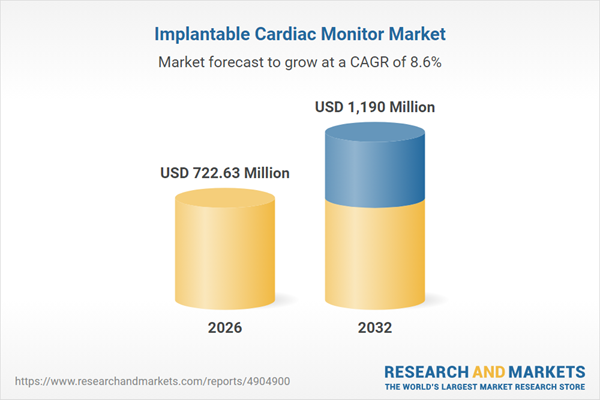
| Estimated Market Value ( USD | $ 722.63 Million |
| Forecasted Market Value ( USD | $ 1190 Million |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 17 |