Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive introduction to in situ hybridization emphasizing scientific foundations, clinical relevance, and its evolving multidisciplinary role
In situ hybridization (ISH) has matured from a specialized laboratory technique into a multidisciplinary tool that underpins critical advances in molecular pathology, translational research, and diagnostic precision. Grounded in the fundamental principle of sequence-specific nucleic acid hybridization, modern ISH workflows combine probe chemistry, imaging modalities, and computational analysis to reveal spatially resolved molecular information at cellular and subcellular scales. This convergence of wet-lab methods and digital analysis has expanded the technique’s relevance beyond classical histology to areas such as single-cell spatial transcriptomics, companion diagnostics, and pathogen localization.Historically deployed to validate gene expression patterns and chromosomal alterations, ISH now supports decision-making across diverse clinical and research contexts. The technique’s capacity to localize DNA and RNA targets directly within tissue sections enhances interpretive clarity relative to bulk assays, enabling clinicians and scientists to link molecular signals to histomorphology. As laboratory automation, probe design, and image analysis software continue to evolve, ISH is increasingly integrated into workflows that demand reproducibility, traceability, and throughput. This integration is particularly important for institutions that must balance rigorous quality control with the need to generate clinically actionable results within constrained timelines.
Consequently, executives and technical leaders should view ISH as a platform technology that interfaces with instruments, reagents, services, and software ecosystems. Its value stems not only from methodological improvements but also from the expanding set of applications that benefit from spatial molecular data. As the field continues to iterate, stakeholders will need to align investments in capital equipment, expert personnel, and informatics to fully realize the technique’s diagnostic and research potential.
Transformative technological and workflow shifts reshaping in situ hybridization through automation, multiplexing, and advanced computational analysis
The landscape of in situ hybridization is undergoing transformative shifts driven by technological convergence, changing clinical demands, and a reorientation toward integrated digital workflows. Advances in probe chemistries and multiplexing strategies have significantly increased the number of targets measurable in a single assay, enabling richer spatial resolution of gene expression patterns. At the same time, automated instrumentation is lowering barriers to adoption by reducing hands-on time, enhancing consistency, and enabling scaling across larger sample volumes. These hardware innovations are complemented by software platforms that provide sophisticated image analysis and data interpretation capabilities, translating raw images into quantifiable biomarkers and standardized outputs.Concurrently, clinical drivers are reshaping use cases for ISH. Precision oncology and targeted therapies require actionable spatial context to guide patient selection, monitor minimal residual disease, and inform combination strategies. Infectious disease diagnostics benefit from rapid, spatially resolved detection of pathogens within tissue architecture, improving understanding of pathogenesis and informing clinical management. In research settings, the push toward single-cell and spatial omics is fueling demand for workflows that can co-register molecular data with histological features, thereby elevating ISH from a confirmatory tool to a discovery platform.
Furthermore, the integration of cloud-enabled analytics and artificial intelligence into ISH pipelines is accelerating adoption by reducing the burden of manual interpretation and enabling reproducible quantitation. These digital enhancements facilitate cross-site collaborations and support remote review, which is particularly important for multi-center trials and decentralized clinical networks. Taken together, these shifts are producing a more interconnected ecosystem in which instrumentation, reagents, services, and software must interoperate seamlessly to deliver consistent, clinically relevant results.
How shifts in tariff policy are influencing procurement, supply chain resilience, and domestic capability development across the in situ hybridization value chain
Policy and trade dynamics, including tariff adjustments, have meaningful operational implications for the ISH ecosystem. Tariffs can alter the cost structure for imported capital equipment, reagents, and specialized consumables, which in turn affects procurement strategies at hospitals, research institutions, and biopharmaceutical companies. When import duties rise, procurement teams often re-evaluate vendor portfolios, prioritize local sourcing where feasible, and extend equipment lifecycles through enhanced maintenance and service agreements to mitigate near-term capital outlays. These shifts can influence purchasing cycles and slow the replacement cadence for both automated systems and manual platforms, prompting laboratories to optimize existing assets through process improvement initiatives.In parallel, tariffs can drive supply chain diversification as vendors and buyers seek to reduce exposure to cross-border policy volatility. Manufacturers may respond by regionalizing production, negotiating alternate logistics routes, or qualifying secondary suppliers for critical reagents such as probes and product kits. Such moves can improve resilience but may increase lead times or require additional validation efforts to ensure batch-to-batch consistency. For service providers that rely on imported materials or instruments, higher operational costs can pressure pricing models and margins, which may be passed on to end users or absorbed temporarily to retain market competitiveness.
Moreover, tariffs can accelerate investment in domestic capabilities, encouraging the development of local reagent production and assembly lines for instruments. This localization trend can create opportunities for new entrants and contract manufacturers, particularly in regions that offer supportive public policy or incentive programs. At the same time, researchers and clinicians may need to adapt to alternative suppliers or modified formulations, necessitating updated validation protocols and quality assurance activities. The net effect of tariff changes is therefore multifaceted: they affect procurement economics, supply chain design, validation burdens, and the strategic priorities of stakeholders across the ISH value chain.
Key segmentation insights linking product classes, probe types, technologies, applications, and end-user needs to strategic adoption dynamics
A nuanced view of segmentation reveals how product classes, technologies, probe types, applications, and end users collectively shape adoption patterns and investment priorities within the ISH domain. When examining product type, instruments encompass both automated systems and manual systems, with automated platforms increasingly preferred in settings that demand consistency and throughput while manual systems retain an essential role in research laboratories and method development. Reagents span probes and product kits, where probe design and specificity are critical determinants of assay performance and interpretation. Services cover consultation services and custom services, reflecting demand for expert assay development, validation, and laboratory outsourcing. Software offerings include data analysis and image analysis tools that convert visual outputs into quantitative metrics, enabling reproducible reporting and downstream biomarker discovery.Looking across technology, chromogenic methods continue to deliver robust, permanent staining options that align with traditional histopathology workflows, whereas fluorescent approaches enable higher multiplexing and compatibility with advanced imaging systems. Probe type differentiates DNA probes and RNA probes, each offering unique strengths: DNA probes remain important for chromosomal localization and copy number assessments, while RNA probes are central to transcript detection and expression profiling at the single-cell level. In application areas, cancer research, genetic disorders, and infectious disease diagnostics represent key use cases where spatial context adds clinical or mechanistic insight, guiding therapeutic decisions and enabling more precise phenotypic characterization.
End-user profiles influence how products and services are configured; hospitals and clinics prioritize validated, regulatory-compliant workflows with streamlined reporting, pharmaceutical and biotech companies emphasize throughput, reproducibility, and compatibility with drug development pipelines, and research laboratories value flexibility for method development and novel assay design. Taken together, these segmentation dimensions interact: for example, a pharmaceutical company pursuing companion diagnostics may select automated instruments, fluorescent technologies, RNA probes, and bespoke software for high-content analysis, while a pathology lab might favor chromogenic assays on validated manual or semi-automated platforms with robust image analysis integration. Understanding these cross-cutting relationships helps stakeholders align product development, service offerings, and commercialization strategies with specific customer needs.
Regional dynamics shaping adoption, regulatory expectations, and commercialization strategies for in situ hybridization across major global markets
Regional dynamics exert a profound influence on technology adoption, regulatory pathways, and commercial strategies for ISH solutions. In the Americas, demand is driven by an established base of academic medical centers, integrated healthcare systems, and a robust life sciences sector that prioritizes translational research and precision oncology initiatives. This environment supports early adoption of automated platforms and advanced analytical tools, while public and private investment in domestic manufacturing can create favorable conditions for localization of reagent and instrument production.Across Europe, the Middle East & Africa, a heterogeneous regulatory and reimbursement landscape shapes deployment strategies. Western European markets often emphasize stringent validation and conformity with regulatory frameworks, which benefits vendors that provide comprehensive documentation and clinical evidence. In contrast, certain markets in the broader region may prioritize cost-effective solutions and partnerships that enable capacity building and technology transfer. Additionally, collaborative networks among academic centers and pan-regional consortia facilitate multi-center studies that leverage ISH for comparative pathology and translational research.
The Asia-Pacific region exhibits a mix of rapid adoption and localized innovation. Large research universities, expanding clinical infrastructures, and growing biotech sectors have increased demand for both instruments and high-quality reagents. Moreover, policy incentives in some countries are fostering domestic manufacturing and R&D capabilities, which can influence procurement preferences and create opportunities for joint ventures or co-development agreements. Across all regions, cross-border collaborations, regulatory harmonization efforts, and investments in digital infrastructure will continue to shape how ISH technologies are adopted and scaled for clinical and research use.
Strategic competitive insights revealing how product innovation, service excellence, and software integration define leadership in the in situ hybridization space
Competitive positioning within the ISH landscape is influenced by differentiated capabilities across instruments, reagent portfolios, service models, and software analytics. Leading instrument providers are investing in automation, modularity, and integration with digital pathology ecosystems to offer turnkey solutions that reduce operational complexity and accelerate time to result. These investments are often paired with expanded service contracts and on-site support offerings that lower adoption barriers for clinical laboratories and high-volume research centers. Reagent developers are focusing on probe specificity, multiplex compatibility, and validated kits that simplify assay implementation while reducing variability.Service providers are distinguishing themselves through bespoke consultation services, assay customization, and contract laboratory offerings that address validation, regulatory submissions, and clinical trial support. The ability to provide end-to-end workflows-from assay design and validation through high-throughput execution and data interpretation-creates strategic value for customers seeking to outsource complex activities or accelerate internal capabilities. Software vendors are concentrating on interoperable platforms that support both data analysis and image analysis, emphasizing user-friendly interfaces, regulatory-ready audit trails, and machine learning-assisted interpretation to improve diagnostic consistency and biomarker discovery.
Partnerships, licensing agreements, and targeted acquisitions continue to be common strategic moves as organizations seek to expand capabilities rapidly without incurring the full cost of in-house development. Intellectual property portfolios, quality management systems, and regulatory track records are key differentiators that influence customer selection, particularly in clinical contexts. For new entrants, focusing on niche applications, strong scientific validation, and collaborative pilots with influential research centers can establish credibility and accelerate adoption. Overall, competitive success derives from aligning technical excellence with robust service delivery and seamless software integration to meet the evolving needs of end users.
Actionable recommendations for leaders to align product innovation, operational scalability, and strategic partnerships to accelerate adoption and resilience
Industry leaders should prioritize an integrated strategy that balances innovation with operational scalability to capture long-term value in ISH. Invest in modular automation that can be scaled across centers of excellence and adapted to evolving multiplexing needs, while ensuring that service networks and training programs are in place to support effective deployment. Parallel investments in high-fidelity probe design and validated reagent kits will reduce variability and accelerate clinical translation, thereby increasing confidence among pathologists and clinical researchers. Moreover, synchronizing instrument roadmaps with software development will enable real-world interoperability, reduce integration friction, and facilitate adoption by institutions with established digital pathology infrastructures.From a commercial perspective, cultivate strategic partnerships with clinical and academic leaders to co-develop assays and generate peer-reviewed evidence that supports clinical utility. Engage early with regulatory and reimbursement stakeholders to streamline validation pathways and align evidence generation with payer requirements. To mitigate supply chain risks, diversify sourcing strategies and consider regional manufacturing partnerships to improve resilience against policy and logistics disruptions. Additionally, offer flexible commercial models-such as reagent-as-a-service, managed testing programs, and bundled software licenses-that align with varied buyer preferences and budget constraints.
Finally, embed data stewardship and AI-readiness into product and service designs to facilitate downstream analytics, regulatory compliance, and cross-institutional studies. Providing clear documentation, version control, and traceable analytics pipelines will enhance trust among clinical users and accelerate integration into diagnostic workflows. These combined actions will position leaders to capture opportunities across research, clinical diagnostics, and drug development applications.
Robust mixed-methods research methodology that integrates primary interviews, secondary validation, and transparent analytical triangulation for reliable insights
The research methodology underpinning this executive summary synthesizes qualitative and quantitative inputs derived from primary interviews, secondary literature, technical white papers, regulatory guidance documents, and validated product specifications. Primary engagement included structured interviews with laboratory directors, pathology specialists, procurement officers, and R&D leads to capture operational realities, adoption criteria, and unmet needs. Secondary sources were systematically reviewed to contextualize technological trends, regulatory developments, and supply chain considerations, with special attention to peer-reviewed studies and technical validations that elucidate assay performance and comparative strengths.Analytical rigor was maintained through cross-validation of claims and triangulation across multiple evidence streams. Device and reagent categorizations were mapped to functional requirements and use case scenarios to ensure recommendations are actionable for diverse stakeholders. Regional dynamics were assessed by examining regulatory frameworks, local manufacturing initiatives, and the composition of end-user institutions to identify pragmatic routes to market and potential barriers to adoption. The methodology emphasized transparency in assumptions and reproducibility of findings, with appendices documenting interview protocols, inclusion criteria for secondary materials, and the logic used to synthesize multi-dimensional segmentation insights.
This approach ensures that the insights presented are grounded in real-world practice, reflect contemporary technological capabilities, and are sensitive to operational constraints faced by clinical and research laboratories. The methodology supports iterative updates and can be adapted to incorporate new primary data or to focus on customized regional or application-specific deep dives.
Concluding synthesis highlighting the strategic convergence of technology, operations, and partnerships necessary to realize the full potential of in situ hybridization
In situ hybridization stands at an inflection point where technical advances, clinical imperatives, and digital transformation are converging to expand its utility across diagnostics and research. The technique’s ability to provide spatially resolved molecular information makes it indispensable for modern pathology and translational science, yet realizing its full potential requires coordinated investments in instruments, validated reagents, expert services, and scalable software. Stakeholders who proactively align these elements will be better positioned to address complex clinical questions, support drug development pipelines, and participate in multi-institutional research networks.Operational resilience, informed procurement strategies, and an emphasis on interoperability will be essential as organizations navigate supply chain uncertainty and evolving regulatory expectations. Moreover, the maturation of image analysis and data interpretation tools will determine how rapidly ISH moves from a specialized technique to a routine component of diagnostic workflows. Ultimately, success in this space will depend on thoughtful integration: aligning scientific rigor with pragmatic deployment strategies, building partnerships that accelerate validation and adoption, and embedding data governance practices that support reproducibility and regulatory acceptance. The opportunity is clear for organizations that can combine technical excellence with strategic execution.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China In Situ Hybridization Market
Companies Mentioned
The key companies profiled in this In Situ Hybridization market report include:- Abbott Laboratories, Inc.
- Abcam plc
- Abnova Corporation
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- Biocare Medical LLC
- Empire Genomics, Inc.
- F. Hoffmann-La Roche AG
- Genemed Biotechnologies, Inc.
- Integrated DNA Technologies, Inc.
- Leica Biosystems Nussloch GmbH
- Merck KGaA
- MetaSystems GmbH
- NanoString Technologies, Inc.
- Oxford Gene Technology Ltd
- PerkinElmer, Inc.
- Thermo Fisher Scientific, Inc.
- Zytomed Systems GmbH
- ZytoVision GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
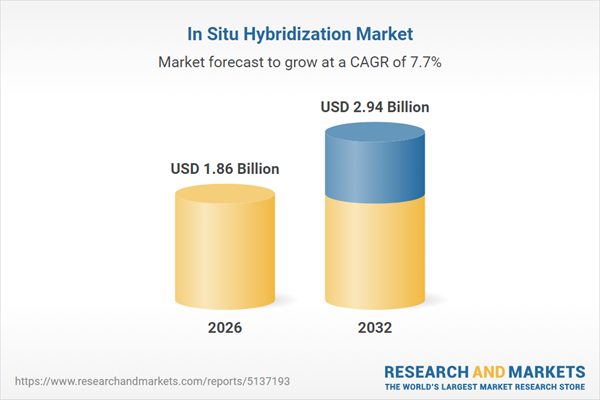
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 1.86 Billion |
| Forecasted Market Value ( USD | $ 2.94 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |