Speak directly to the analyst to clarify any post sales queries you may have.
An integrated overview of instrument classes, clinical needs, and configuration imperatives shaping modern in-vitro diagnostics strategies
The in-vitro diagnostics (IVD) instrument landscape is undergoing substantive evolution as technological advances, shifting clinical priorities, and supply chain considerations converge. Instrumentation now spans traditional analyzers and emergent point-of-care devices, and understanding this breadth is essential for stakeholders seeking to align product development with clinical and operational demand. Instruments such as chemistry analyzers, coagulation systems, hematology platforms, immunoassay instruments, microbiology analyzers, molecular diagnostics platforms, and point-of-care devices each present distinct development pathways, regulatory touchpoints, and reimbursement dynamics that influence commercialization strategies.Across clinical applications, diagnostic priorities range from chronic disease monitoring to acute infectious disease detection. Autoimmune testing, cardiac biomarker panels, diabetes monitoring, infectious disease assays, and oncology diagnostics drive differing requirements around throughput, sensitivity, sample type compatibility, and turn-around time. Technology choices further shape instrument design and performance. Chromatography, cytometry, immunoassay, microscopy, molecular methods, and spectrometry underpin assay performance, with subtypes such as affinity or liquid chromatography, flow or image cytometry, chemiluminescent or ELISA immunoassays, confocal or electron microscopy, hybridization and next-generation sequencing in molecular workflows, and mass or fluorescence spectrometry in analytical pipelines.
End users-academic research centers, diagnostic laboratories, and hospitals-impose operational requirements that influence form factor and configuration decisions, driving demand for benchtop, floor-standing, handheld, and portable systems. This introduction synthesizes the interplay between instrument taxonomy, clinical application, and deployment environment, setting the stage for deeper examination of market shifts and strategic implications for manufacturers, laboratory directors, and clinical leaders.
How technological convergence, decentralization of testing, and digital integration are redefining the operational and clinical landscape of diagnostic instrumentation
The IVD instrumentation landscape is experiencing transformative shifts driven by technological maturation, changing care delivery models, and heightened expectations for speed and precision. Advances in molecular technologies, especially next-generation sequencing and high-throughput PCR platforms, are driving increased adoption of molecular diagnostics beyond niche applications; these platforms are increasingly incorporated into routine workflows for infectious disease detection, oncology profiling, and precision medicine initiatives. Immunoassay technologies continue to evolve as chemiluminescence and high-sensitivity fluorescence methods deliver improved signal-to-noise performance, enabling earlier detection and more reliable longitudinal monitoring for conditions such as cardiac events and autoimmune disorders.Simultaneously, the rise of point-of-care testing is reshaping how and where diagnostics are performed. Portable and handheld configurations are converging with robust analytic capabilities, enabling rapid decision-making in emergency departments, outpatient clinics, and decentralized care settings. This decentralization is complemented by digital integration, as instruments increasingly embed connectivity for laboratory information systems, cloud-based analytics, and remote device management; such integration supports operational efficiency and data-driven quality control. Flow and image cytometry are expanding in both research and clinical spheres, offering deeper cellular phenotyping that supports personalized therapy selection, while advancements in spectrometry and chromatography are expanding the analytical reach of clinical laboratories.
Operationally, diagnostic laboratories and hospitals are adapting to these technological shifts by redesigning workflows to accommodate multiplexed testing and by investing in automation to reduce manual intervention and turnaround times. Academic research entities are leveraging advanced microscopy and molecular platforms to accelerate translational research, creating feedback loops between innovation and clinical deployment. As these shifts unfold, manufacturers must prioritize modularity, interoperability, and regulatory agility to remain competitive, while health systems must balance capital investment with demonstrable clinical value and improved patient outcomes.
Assessing the operational ramifications of 2025 United States tariff measures on supply chain resilience, sourcing strategies, and clinical procurement practices
Trade policy developments and tariff regimes can materially affect the IVD instrument ecosystem by altering supply chain economics, procurement strategies, and regional manufacturing decisions. Tariffs implemented by the United States in 2025 have created measurable friction for manufacturers and distributors that rely on cross-border components, specialized consumables, and assembly partnerships. Increased import duties on key instrument components have led some organizations to re-evaluate sourcing strategies, including diversification of supplier bases and the relocation of select manufacturing steps to mitigate exposure to tariff-related cost increases.The cumulative impact extends beyond unit cost considerations: tariffs have prompted a reexamination of lead times, inventory strategies, and contractual terms with suppliers, particularly for components with limited substitutes or long qualification timelines. Many instrument manufacturers have moved toward dual sourcing for critical electronic components, optics, and reagent substrates while negotiating longer-term supply contracts to lock in pricing stability. Laboratory purchasers and healthcare systems have responded by adjusting procurement cadences and prioritizing instruments and consumables with established local supply chains to minimize disruption.
In addition, tariff-driven adjustments have influenced decisions around capital deployment and after-sales support. Some manufacturers have increased local service operations and spares inventories to preserve uptime in high-demand clinical settings. Regulatory compliance and customs classification complexities have required enhanced trade compliance expertise within companies, leading to investments in customs optimization and tariff engineering where legally permissible. Collectively, these adaptations underscore the role of trade policy as a strategic variable in operational planning for manufacturers, distributors, and end users, shaping decisions that will influence resilience and competitiveness into the mid-term.
Segment-tailored instrument strategies that align technology choices, application priorities, and configuration preferences to end-user operational realities
Segment-specific dynamics reveal differentiated opportunities and constraints across instrument type, application, technology, end-user, and configuration axes. Within instrument type, chemistry analyzers and hematology platforms remain core laboratory workhorses, while immunoassay analyzers and molecular diagnostics systems increasingly capture attention for their role in precision testing and targeted therapeutics. Microbiology analyzers are adapting to faster pathogen identification needs, and point-of-care devices are expanding into routine screening and ambulatory care, reshaping care pathways.Application-level demand influences instrument specifications and service models. Cardiac marker testing and diabetes management require reliable high-throughput solutions with rapid turnaround, whereas infectious disease diagnostics and oncology profiling demand high sensitivity, specificity, and often molecular-level resolution. Autoimmune testing places premium importance on assay reproducibility and longitudinal comparability. These application demands cascade into technology selection, with chromatography and spectrometry methods serving complex analytical chemistry needs, cytometry enabling detailed cellular analysis, immunoassays offering robust protein detection, microscopy providing morphological insights, and molecular techniques delivering nucleic acid-level resolution.
End-user requirements further stratify instrument deployment strategies. Academic research institutions prioritize flexibility and advanced feature sets to support exploratory assays and method development, diagnostic laboratories focus on throughput, cost per test, and automation to achieve consistent operations, and hospitals demand integrated solutions that support rapid clinical decision-making at the point of care. Configuration preferences-benchtop, floor-standing, handheld, and portable-reflect trade-offs between footprint, throughput, and mobility. Manufacturers that align product roadmaps with these nuanced segmentation vectors-designing modular platforms that scale from benchtop to floor-standing or offering handheld variants with validated performance-will be better positioned to meet the evolving needs of diverse clinical and research customers.
Regional dynamics and regulatory nuances shaping adoption patterns, procurement approaches, and supply chain choices across the Americas EMEA and Asia-Pacific
Regional dynamics shape investment, adoption patterns, and supply chain choices across the Americas, Europe, Middle East & Africa, and Asia-Pacific, with each region presenting distinct regulatory, clinical, and economic considerations. In the Americas, healthcare systems emphasize rapid diagnostic turnaround and integration with electronic medical records, driving demand for automated analyzers and connected point-of-care solutions. Procurement practices in this region often balance centralized laboratory efficiency with growing decentralized testing needs, particularly in ambulatory and urgent care settings.Across Europe, the Middle East & Africa, regulatory harmonization in parts of Europe encourages cross-border product deployment, while fragmented reimbursement environments and variable access in the Middle East and Africa require adaptable commercial strategies. Clinical laboratories in these markets often prioritize robust quality management systems and compliance features, influencing instrument selection and aftermarket service models. In the Asia-Pacific region, rapid investment in healthcare infrastructure, combined with high-volume testing needs and a strong manufacturing base, creates an environment conducive to both local production and rapid adoption of innovative molecular and immunoassay platforms. Supply chain decisions in this region are often guided by proximity to component suppliers and reagent manufacturers, as well as by national strategies to bolster diagnostic capacity.
Manufacturers and distributors should calibrate go-to-market strategies to regional nuances, balancing centralized manufacturing with regional assembly or local partnerships to optimize cost and responsiveness. Regulatory strategy must be regionally informed, given differing approval pathways and conformity requirements. End users across regions will continue to prioritize reliability, total cost of ownership, and integrated digital solutions, making region-specific service networks and training programs essential components of a successful commercial strategy.
Competitive differentiation driven by modular platforms, digital connectivity, reagent control, and localized service capabilities across the diagnostics instrument landscape
Competitive positioning in the IVD instrument space is defined by a combination of technological leadership, breadth of portfolio, and global service capabilities. Leading firms differentiate through investments in modular platforms that enable seamless upgrades and through strategic partnerships that expand assay menus and extend clinical utility. Companies that combine strong channel networks with localized service infrastructure gain a competitive advantage by reducing downtime and improving user adoption rates, particularly in high-acuity hospital environments and high-throughput diagnostic laboratories.Innovation leaders are those that integrate digital analytics and connectivity into instrument platforms, enabling predictive maintenance, remote troubleshooting, and performance benchmarking across laboratory networks. These capabilities not only support operational efficiency but also create opportunities for value-added services such as centralized quality assurance and real-world evidence generation. Strategic M&A and collaborative development agreements continue to shape the competitive landscape, allowing companies to accelerate entry into adjacent technologies such as next-generation sequencing workflows or advanced mass spectrometry applications.
Supply chain resilience and reagent ecosystem control are additional competitive levers. Firms with vertically integrated reagent production and secure distribution channels can better manage pricing pressures, mitigate supply disruptions, and ensure consistent assay performance. As customers increasingly evaluate total cost of ownership and long-term service commitments, companies that offer transparent lifecycle management, comprehensive training, and flexible financing or leasing options will strengthen commercial relationships and expand adoption across hospitals, diagnostic labs, and academic centers.
Practical strategic actions for manufacturers and service providers to accelerate adoption, strengthen resilience, and deliver measurable clinical and operational value
Leaders in the diagnostics instrument sector should pursue a set of pragmatic actions to capitalize on technological momentum while mitigating operational risks. First, prioritize modular product architectures that allow for incremental upgrades of optics, molecular modules, or software analytics without full platform replacements. This approach reduces capital burden for customers and shortens product development cycles. Second, invest in cloud-enabled connectivity and remote diagnostics capabilities to provide customers with predictive maintenance, usage analytics, and streamlined software updates that reduce downtime and support continuous quality improvement.Third, diversify supply chains for critical components and consider regionalized manufacturing or assembly hubs to mitigate tariff exposure and reduce lead times. Establishing dual sourcing strategies and maintaining strategic safety stocks for critical consumables will enhance resilience. Fourth, expand assay menus through strategic partnerships that accelerate access to high-value applications such as oncology profiling and infectious disease multiplexing; co-development agreements can fast-track clinically validated assays while sharing commercial risk. Fifth, align commercial models with customer procurement realities by offering flexible financing, managed service agreements, and outcomes-oriented pricing where feasible, which can lower barriers to adoption for resource-constrained laboratories and hospitals.
Finally, enhance regulatory and trade compliance capabilities to proactively address tariff impacts and product registration complexities. Building strong local training and service ecosystems will differentiate offerings and improve clinical adoption. By executing these recommendations with disciplined project management and clear ROI articulation, companies can strengthen market positioning and deliver sustained value to clinical and research customers.
A rigorous mixed-methods approach combining expert interviews, technical validation, and cross-checked secondary evidence to ensure analytical credibility and practical relevance
The research underpinning this analysis combined a multi-pronged methodology that integrates primary interviews, targeted secondary review, and systematic synthesis to ensure robust, actionable findings. Primary research included structured interviews with laboratory directors, hospital procurement leads, clinical pathologists, and product managers from instrument manufacturers to capture first-hand insights into technology adoption drivers, procurement considerations, and service expectations. These qualitative data points were augmented by technical discussions with subject matter experts in molecular diagnostics, clinical chemistry, cytometry, and microbiology to validate technology-specific observations and performance trade-offs.Secondary research drew on peer-reviewed literature, regulatory filings, clinical guidelines, and manufacturer technical documentation to corroborate capability claims and to understand regulatory and compliance implications. Publicly available clinical studies and validation reports were assessed to evaluate performance characteristics of key technologies such as next-generation sequencing, PCR-based molecular assays, immunoassays, and advanced spectrometry. Data triangulation was employed to reconcile potential inconsistencies between primary insights and secondary evidence, ensuring findings reflect both practitioner experience and empirical performance metrics.
Analytical rigor was maintained through cross-validation of supplier claims, sensitivity analysis of operational impacts such as turnaround time reductions and workflow efficiencies, and careful consideration of regional regulatory variances. Limitations of the methodology include potential selection bias inherent in expert interviews and the evolving nature of technology adoption curves, which can introduce variability in adoption timelines. Nonetheless, the combined qualitative and technical approach provides a credible foundation for the strategic observations and recommendations presented.
Synthesis of technological momentum, operational imperatives, and strategic priorities shaping the next generation of diagnostic instrument deployment
The current era for in-vitro diagnostics instruments is characterized by rapid technological maturation, shifting clinical workflows, and strategic supply chain recalibration. Molecular and immunoassay innovations, enhanced digital integration, and the expansion of point-of-care capabilities are collectively raising clinical expectations for faster, more precise diagnostics that better inform patient management. At the same time, trade policy adjustments, including tariff measures, have elevated the importance of supply chain diversification and regional manufacturing considerations, prompting manufacturers and purchasers to rethink procurement and service models.Stakeholders that proactively align product roadmaps with clinical application priorities, invest in modular and connected platforms, and develop resilient supply and service ecosystems will capture the greatest strategic advantage. Regional nuances in regulation, reimbursement, and infrastructure necessitate tailored commercial strategies, while end users increasingly evaluate total cost of ownership, uptime guarantees, and assay breadth when making procurement decisions. By focusing on interoperability, assay agility, and lifecycle support, manufacturers can better meet the complex demands of hospitals, diagnostic laboratories, and academic institutions. The confluence of these forces points toward a diagnostics ecosystem that is more connected, more adaptable, and more integrated with clinical decision-making than ever before.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China In-Vitro Diagnostics Instrument Market
Companies Mentioned
The key companies profiled in this In-Vitro Diagnostics Instrument market report include:- Abbott Laboratories
- Agilent Technologies, Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- BioMérieux S.A.
- Danaher Corporation
- EKF GmbH
- F. Hoffmann-La Roche Ltd
- Hologic, Inc.
- Illumina, Inc.
- Mesolonghi Diagnostics
- Ortho Clinical Diagnostics
- PerkinElmer, Inc.
- PHC Corporation
- Qiagen N.V.
- Roche Diagnostics
- Siemens Healthineers AG
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Tulip Diagnostics (P) Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
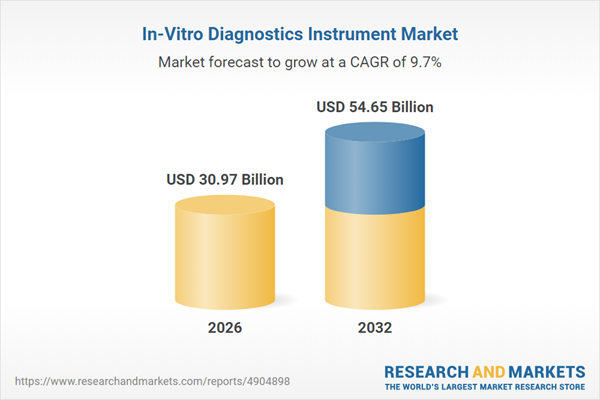
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 30.97 Billion |
| Forecasted Market Value ( USD | $ 54.65 Billion |
| Compound Annual Growth Rate | 9.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |