Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Increasing Prevalence of Neurological Disorders, Especially Stroke
The rising burden of neurological disorders - particularly stroke - remains a core driver of the India Neurovascular Devices Market. With an estimated prevalence ranging from 3.0 to 11.9 cases per 1,000 individuals, neurological illnesses are placing mounting pressure on India’s healthcare infrastructure. A study published in The Lancet Neurology revealed that annual stroke incidence more than doubled from 650,000 cases in 1990 to over 1.25 million in 2021.This rise is attributed to common risk factors such as hypertension, diabetes, sedentary lifestyles, and increasing life expectancy. Importantly, strokes are now increasingly affecting younger and middle-aged adults in both urban and semi-urban populations. As a result, the need for scalable, interventional medical solutions is more urgent than ever. This has significantly boosted procedural demand for thrombectomy, stenting, embolization, and aspiration techniques - thereby accelerating the use of devices like stent retrievers, microcatheters, and flow diverters across Indian hospitals.
Key Market Challenges
High Cost of Neurovascular Devices and Limited Insurance Coverage
A major constraint for the market is the high cost associated with advanced neurovascular devices, most of which are imported. Technologies such as stent retrievers, flow diverters, embolization coils, and thrombectomy catheters come with premium pricing, which increases the financial burden on patients. Limited insurance coverage for these high-cost procedures restricts accessibility and poses a challenge for widespread adoption, particularly in smaller hospitals and public health settings.Key Market Trends
Expansion of Neuro-Interventional Infrastructure in Tier 2 and Tier 3 Cities
While neurovascular procedures were previously concentrated in large private hospitals in major metropolitan areas, there is now a shift toward expanding such infrastructure in Tier 2 and Tier 3 cities. Public and private stakeholders are actively setting up stroke care units, neuroimaging facilities, and interventional radiology departments in secondary urban centers and medical colleges. This trend is increasing the installed base for neurovascular equipment and improving access to neurological care in underserved regions. Additionally, as clinical training initiatives expand and device manufacturers establish regional partnerships, device adoption is expected to grow substantially in semi-urban and smaller cities.Key Market Players
- Stryker
- Medtronic
- Terumo India Private Limited
- Medical Device Business Services, Inc
- Penumbra, Inc.
- B. Braun SE
- Integra LifeSciences Corporation
- Acandis India Pvt. Ltd
- MicroPort Scientific Corporation
Report Scope:
In this report, the India Neurovascular Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:India Neurovascular Devices Market, By Device:
- Cerebral Embolization and Aneurysm Coiling Devices
- Cerebral Angioplasty and Stenting Systems
- Neurothrombectomy Devices
- Support Devices
- Trans Radial Access Devices
India Neurovascular Devices Market, By Therapeutic Application:
- Stroke
- Cerebral Artery
- Cerebral Aneurysm
- Others
India Neurovascular Devices Market, By Therapeutic End User:
- Hospitals
- Specialty Clinics
- Others
India Neurovascular Devices Market, By Region:
- North India
- East India
- West India
- South India
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the India Neurovascular Devices Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Stryker
- Medtronic
- Terumo India Private Limited
- Medical Device Business Services, Inc
- Penumbra, Inc.
- B. Braun SE
- Integra LifeSciences Corporation
- Acandis India Pvt. Ltd
- MicroPort Scientific Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 85 |
| Published | July 2025 |
| Forecast Period | 2024 - 2030 |
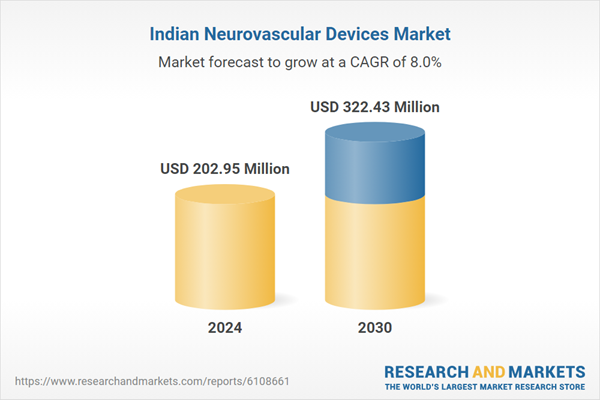
| Estimated Market Value ( USD | $ 202.95 Million |
| Forecasted Market Value ( USD | $ 322.43 Million |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | India |
| No. of Companies Mentioned | 9 |