Speak directly to the analyst to clarify any post sales queries you may have.
Establishing the Framework for Modern Infusion Pump Ecosystem Evolution Driven by Technological Advancements and Shifting Healthcare Service Requirements
The infusion pump industry stands at the nexus of healthcare delivery and technological innovation, propelled by the imperative to enhance patient safety, treatment accuracy, and operational efficiency. Over the past decade, infusion pumps have evolved from mechanical devices to intelligent systems, integrating sensor technology, wireless connectivity, and data analytics. As healthcare providers strive to optimize therapeutic regimens for complex conditions, the demand for precision infusion solutions continues to rise.Furthermore, the shift toward outpatient care and home-based therapies has catalyzed the development of portable and user-friendly devices. Stakeholders now require insights into how these trends reshape purchasing decisions, regulatory compliance, and patient adherence. The proliferation of chronic diseases and an aging demographic exacerbate resource constraints in hospitals, underscoring the need for scalable infusion technologies. Consequently, manufacturers, payers, and care providers must align on interoperable platforms, robust cybersecurity protocols, and intuitive user interfaces.
Building on this landscape, this executive summary frames the key technological, clinical, and policy drivers influencing infusion pump adoption. It offers a cohesive narrative that synthesizes industry dynamics and sets the stage for deeper analyses of tariff implications, segmentation nuances, regional dynamics, and competitive positioning. Through this framework, readers will grasp the essential context necessary for informed decision-making and strategic planning in a rapidly evolving ecosystem.
Unveiling Paradigm Altering Transformations Redefining Infusion Pump Landscape Under the Influence of Digital Integration, Smart Connectivity, and Personalized Therapy Trends
The infusion pump landscape is experiencing transformative shifts as digital health integration and personalized medicine converge. Connected pump platforms now transmit real time dosing data into electronic health records, enabling clinicians to tailor infusion regimens based on patient variability and clinical outcomes. This fusion of connectivity and analytics is redefining best practices in medication safety and therapeutic optimization.Simultaneously, stakeholders are exploring mobile health applications and cloud based dashboards that facilitate remote monitoring of infusion therapy. Such innovations reduce the burden on inpatient facilities, support telehealth consultations, and enhance patient engagement. Interoperability standards are maturing, allowing seamless communication between pumps, smart hospital beds, and decision support systems.
Moreover, the emergence of closed loop insulin delivery and chemotherapy infusion protocols exemplify the potential of automation in critical care. These developments portend a shift from reactive pump management to proactive, predictive maintenance and dosing adjustments. Consequently, manufacturers are reengineering product roadmaps to incorporate artificial intelligence and machine learning algorithms, positioning infusion pumps as integral components of next generation precision medicine ecosystems.
Analyzing the Comprehensive Impact of United States Tariffs on Infusion Pump Supply Chains, Pricing Structures, and Global Competitiveness in 2025
The imposition of new United States tariffs in 2025 has introduced significant variables into global supply chains for infusion pump components and finished devices. Many manufacturers source key elements such as sensors, microprocessors, and specialized polymers from tariff targeted regions, driving up procurement costs and compelling reevaluation of supplier diversification strategies. As transitional measures, firms are exploring onshoring initiatives and nearshore partnerships to mitigate exposure to elevated import duties.In addition, tariff-related cost increases have influenced pricing negotiations with healthcare providers and government agencies. To preserve margin integrity, some organizations are shifting toward value based contracting models that emphasize total cost of therapy rather than unit pricing. This approach aligns incentives between manufacturers and payers by tying reimbursement to clinical outcomes and device utilization metrics.
Simultaneously, the tariff environment has accelerated strategic alliances aimed at localizing manufacturing footprints. Joint ventures and contract manufacturing expansions in the Americas seek to circumvent duty escalations while strengthening resilience against future policy shifts. As a result, market participants are recalibrating logistics frameworks and inventory management practices to optimize lead times and reduce working capital demands.
In-Depth Segmentation Exploration Revealing Insights by Product Type, Administration Mode, Category, Application, End User, and Distribution Channel
A granular segmentation approach reveals differentiated dynamics across the infusion pump spectrum. Product type variation notably shapes adoption patterns: elastomeric infusion pumps, syringe infusion pumps, and volumetric infusion pumps serve distinct clinical protocols, dosing precision needs, and setting requirements. Elastomeric devices are prized for their simplicity and portability in field environments, whereas syringe pumps facilitate high accuracy delivery in neonatal and pediatric care. Volumetric pumps, with larger fluid reservoirs and advanced infusion profiles, are predominant in hospital wards and intensive care units.The dichotomy of continuous infusion versus intermittent administration further delineates therapeutic utility. Continuous pumps support steady state dosing for critical medications such as insulin and analgesics, while intermittent pumps enable bolus delivery schedules, often employed in chemotherapy regimens. Portable infusion pumps contrast with stationary units, offering mobility for patients who transition between homecare settings and outpatient clinics, whereas stationary pumps remain integral to in-hospital infusion suites.
Clinical applications span anesthesia, oncology therapy, pain management, and parenteral nutrition, each demanding unique safety features, software interfaces, and compliance protocols. End user environments ranging from ambulatory care centers and clinics to homecare settings and hospitals require adaptable device designs, training programs, and service models. Distribution channels, including hospital pharmacies, online pharmacies, and retail pharmacies, influence procurement cycles and device availability, underscoring the importance of integrated supply chain strategies to meet end-user demands efficiently.
Dissecting Regional Dynamics Shaping Infusion Pump Demand and Adoption Across the Americas Europe Middle East Africa and Asia Pacific Markets
Regional dynamics exert a profound influence on infusion pump adoption and innovation pathways. In the Americas, robust healthcare infrastructure and proactive reimbursement policies support rapid uptake of advanced connectivity features and patient monitoring integrations. Initiatives to expand home infusion services and telehealth reimbursement have further propelled demand for portable pump technologies, particularly within chronic disease management programs.Europe, the Middle East & Africa exhibit diverse regulatory landscapes and varying levels of healthcare modernization. Western European markets prioritize interoperability and cybersecurity compliance, aligning with stringent regulatory directives. Meanwhile, emerging economies in the Middle East and Africa demonstrate selective adoption driven by investment in tertiary care facilities and partnerships with global device manufacturers.
In the Asia-Pacific region, surging healthcare expenditures and expanding hospital networks underpin interest in cost effective infusion solutions. Government led healthcare reforms emphasize digital health ecosystems and remote patient monitoring, stimulating demand for infusion pumps with integrated cloud capabilities. Concurrently, local manufacturers are scaling production capacities to cater to both domestic and export markets, intensifying competition while driving regional innovation hubs.
Profiling Leading Infusion Pump Manufacturers and Innovators Driving Competitive Differentiation Through Technology Portfolio Diversity and Strategic Partnerships
Industry leaders are differentiating through continuous product innovation, strategic collaborations, and comprehensive service offerings. Major medical device manufacturers have accelerated R&D investments to embed advanced patient safety algorithms, wireless connectivity protocols, and intuitive user interfaces into their infusion pump portfolios. Partnerships with software vendors and telecom providers are enabling enriched data analytics services, predictive maintenance frameworks, and real-time clinical decision support integrations.Several innovators are exploring modular platform architectures that allow rapid feature upgrades and customization for specific therapy areas. This approach reduces time to market for new functionalities and creates opportunities for recurring revenue through software as a medical device. Additionally, OEMs are forging alliances with contract manufacturers and regional assemblers to localize production, optimize costs, and enhance supply chain agility.
Moreover, service providers are offering comprehensive pump fleet management solutions that encompass device tracking, preventative maintenance scheduling, and end-user training programs. These integrated service models aim to minimize downtime, ensure regulatory compliance, and improve total cost of ownership for healthcare institutions. By leveraging digital twin simulations and machine learning algorithms, leading companies are driving continuous performance improvements and fostering stronger customer partnerships.
Crafting Actionable Strategic Recommendations to Propel Infusion Pump Industry Leaders Toward Sustainable Growth Competitive Advantage and Patient Centric Innovation
To sustain growth and maintain competitive advantage, industry leaders should prioritize integration of secure digital ecosystems that connect infusion pumps with broader hospital information systems. Investing in modular software architectures will facilitate rapid deployment of new clinical protocols and regulatory updates without hardware redesign cycles. Strategic collaborations with telehealth providers and analytics specialists can unlock value based contracting opportunities and support outcome driven reimbursement models.Additionally, organizations must diversify supply chain networks by establishing regional manufacturing hubs and building strategic inventory buffers. This approach will mitigate exposure to policy driven tariff fluctuations and global logistics disruptions. Implementing predictive maintenance programs with remote diagnostics capabilities can reduce unplanned downtime and optimize device utilization, thereby enhancing overall service lifecycle management.
Furthermore, expanding training and support services for healthcare professionals and caregivers will drive higher adoption rates and improve patient outcomes. Tailored education programs and virtual training platforms can accelerate proficiency and foster end user confidence in advanced infusion technologies. By adopting a patient centric mindset and aligning commercial strategies with clinical objectives, manufacturers can differentiate their offerings and secure long term partnerships across varied care settings.
Outlining Rigorous Research Framework and Methodological Approach Underpinning the Infusion Pump Market Analysis with Multi Modal Data Validation Techniques
This research employs a multi modal methodology combining primary and secondary data sources to ensure comprehensive and accurate analysis. Primary research included in depth interviews with key opinion leaders, infusion pump clinical specialists, procurement executives at healthcare institutions, and regulatory authorities. These conversations provided qualitative insights into adoption drivers, reimbursement environments, and emerging technological requirements.Secondary research encompassed review of peer reviewed journals, industry white papers, regulatory filings, and corporate disclosures to contextualize market developments and competitive strategies. Data triangulation techniques were applied to validate findings and reconcile any discrepancies between sources. Quantitative analytics were conducted on historical device adoption trends, import-export volumes, and tariff schedules to elucidate supply chain impacts.
Furthermore, scenario analysis was performed to assess strategic implications of potential policy shifts and technological breakthroughs. Expert panels reviewed preliminary findings and provided critical feedback, enhancing the robustness of conclusions and recommendations. This rigorous framework ensures that the insights presented are both actionable and reflective of real world market dynamics.
Summarizing Key Findings and Concluding on the Future Trajectory of the Infusion Pump Sector Amidst Technological and Regulatory Headwinds
In conclusion, the infusion pump sector is undergoing a profound transformation driven by digital integration, patient centric care models, and evolving policy landscapes. The 2025 United States tariffs have underscored the necessity for diversified supply chains and strategic regional manufacturing investments. Segmentation analysis highlights differentiated demand across product types, administration modes, care settings, and distribution channels, necessitating tailored strategies for each cohort.Regional insights reveal that while mature markets in the Americas and Western Europe are accelerating adoption of connected infusion platforms, emerging regions are poised for sustained growth fueled by healthcare modernization initiatives. Competitive dynamics are intensifying as manufacturers innovate modular architectures, augment service portfolios, and pursue collaborative ventures to embed infusion pumps within broader digital health ecosystems.
For industry leaders, the path forward involves harmonizing technology development with regulatory compliance, forging partnerships that support value based care models, and deploying agile supply chain strategies. By embracing these imperatives, organizations can navigate regulatory headwinds, optimize device performance, and deliver superior patient outcomes in an increasingly complex global environment.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Infusion Pumps Market
Companies Mentioned
The key companies profiled in this Infusion Pumps market report include:- 3M Company
- Abbott Laboratories
- Avanos Medical, Inc.
- B. Braun SE
- Baxter International Inc.
- Bayer AG
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Canadian Hospital Specialties Limited
- Chemyx Inc.
- Epic Medical Pty Ltd.
- Fresenius Se & Co. KGaA
- FUJIFILM Corporation
- GE HealthCare Technologies Inc.
- ICU Medical, Inc.
- Insulet Corporation
- Intera Oncology, Inc.
- Intuvie Holdings LLC
- Ivenix, Inc.
- JMS Co., Ltd.
- Longer Precision Pump Co. Ltd. by Halma PLC
- Medline Industries, LP
- Medtronic PLC
- Merck KGaA
- Micrel Medical Devices SA
- Moog Inc.
- Narang Medical Limited
- Nipro Corporation
- Shenzhen MedRena Biotech Co., Ltd.
- SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD.
- Siemens AG
- SOOIL Developments Co., Ltd.
- Tandem Diabetes Care, Inc.
- Teleflex Incorporated
- Terumo Corporation
- Thermo Fisher Scientific Inc.
- Ypsomed AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
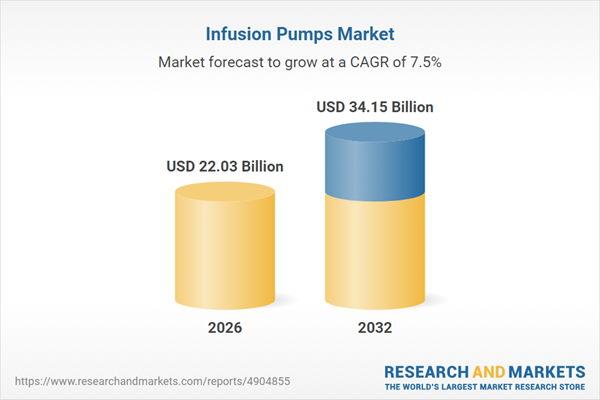
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 22.03 Billion |
| Forecasted Market Value ( USD | $ 34.15 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 38 |