Speak directly to the analyst to clarify any post sales queries you may have.
Clear framing of the intrauterine device landscape that connects clinical practice, policy drivers, and technology trends to strategic decision-making
Intrauterine devices (IUDs) occupy a critical intersection of clinical effectiveness, patient preference, and healthcare system planning, prompting stakeholders to reassess product portfolios, service models, and supply chain strategies. This introduction frames the report's scope by outlining the current clinical landscape, technological advances, and evolving health-policy drivers that shape product adoption and program implementation. It foregrounds how clinician practice patterns, patient counseling priorities, and reimbursement frameworks interact to influence selection between device modalities and durability profiles.
The narrative begins with clinical indications and patient-centered considerations, then transitions to device innovation and regulatory pathways that affect device introductions and post-market surveillance. It highlights how cross-disciplinary collaboration among obstetrics-gynecology, family planning, and primary care is increasingly necessary to translate clinical evidence into routine practice. By situating devices within healthcare delivery realities, this introduction prepares stakeholders to interpret subsequent sections with an eye toward practical decisions in procurement, portfolio strategy, and service design.
How simultaneous advances in device technology, care delivery innovation, and regulatory oversight are reshaping access, adoption, and competitive dynamics
The landscape for intrauterine devices is undergoing transformative shifts driven by technological refinement, enhanced patient autonomy, and changes in care delivery models. Advances in device materials and hormonal delivery profiles are enabling clinicians to offer more tailored contraceptive options that align with diverse patient goals, while smaller-profile insertion systems and extended-duration options reduce procedural complexity and revisit rates. These product-level improvements coincide with evolving clinical guidelines that emphasize informed choice and shared decision-making, which in turn heighten the importance of patient education and clinician training.
Simultaneously, digital health platforms and telemedicine have reshaped pre-procedural counseling and follow-up care, allowing providers to triage suitability and monitor outcomes remotely. Supply chain innovation and alternative distribution channels are expanding access beyond traditional hospital settings, prompting manufacturers and distributors to rethink service models and inventory strategies. Regulatory scrutiny and improved post-market surveillance mechanisms are also recalibrating product lifecycle management, making iterative design changes and real-world evidence generation essential for long-term competitiveness. These converging shifts create both opportunity and complexity, requiring stakeholders to balance rapid innovation with rigorous clinical validation and scalable deployment pathways.
The multifaceted consequences of 2025 tariff changes that compelled supply chain resilience, regional production, and contractual re-evaluation across stakeholders
The imposition of tariffs and trade policy adjustments in 2025 introduced a new strategic variable for suppliers, distributors, and health systems operating across multiple jurisdictions. Elevated import duties on medical goods and related components increased procurement sensitivity and prompted procurement teams to re-evaluate sourcing strategies, inventory buffers, and supplier diversification. In response, manufacturers accelerated nearshoring and regionalization initiatives to mitigate tariff exposure while preserving continuity of supply for procedural settings and clinics that depend on consistent device availability.
Tariff-induced cost pressures also influenced contract negotiations between manufacturers and large institutional purchasers, encouraging more sophisticated total cost of ownership analyses that factor in logistics, compliance costs, and inventory carrying. Clinical stakeholders experienced indirect effects as hospitals and ambulatory centers examined procedural scheduling and device selection to manage consumable costs without compromising quality. Regulatory and compliance teams saw increased workload as customs classifications and import documentation demanded closer coordination across legal, quality, and supply-chain functions. Overall, the cumulative effect of trade policy changes in 2025 drove strategic shifts toward supply chain resilience, local production partnerships, and a stronger emphasis on contractual flexibility across the value chain.
Integrated segmentation perspective revealing how device type, distribution channel, end user, and usage duration jointly determine adoption pathways and service requirements
Segmentation analysis reveals differentiated dynamics across device attributes, distribution vectors, clinical settings, and intended duration of use, each carrying distinct implications for adoption and service delivery. Based on Device Type, market is studied across Copper and Hormonal, where device selection reflects both clinical contraindications and patient preference for hormonal modulation versus non-hormonal options. Based on Distribution Channel, market is studied across Hospital Pharmacies, Online Pharmacies, and Retail Pharmacies, with each channel presenting unique inventory management, regulatory compliance, and counseling touchpoint challenges that affect uptake and patient experience. Based on End User, market is studied across Ambulatory Surgical Centers, Hospitals, and Specialty Clinics, which differ in procedural throughput, staffing expertise, and capital allocation for device inventory. Based on Usage Duration, market is studied across Over Five Years, Three To Five Years, and Under Three Years, where duration profiles influence both patient counseling and long-term follow-up pathways.
Integrating these segmentation lenses exposes where demand concentration and clinical fit converge. Device type and usage duration often correlate, with certain hormonal formulations optimized for extended duration and copper devices frequently positioned as long-term non-hormonal solutions. Distribution channel choice shapes educational touchpoints; for example, online pharmacies expand accessibility but require robust telehealth links to ensure proper counseling, whereas hospital pharmacies embed devices within procedural workflows but face higher cost and inventory constraints. End-user segmentation highlights that ambulatory surgical centers and specialty clinics can drive rapid adoption of innovative insertion technologies due to focused procedural volumes and specialized staff, while hospitals prioritize standardization and cross-departmental compatibility. This layered perspective helps stakeholders align product design, distribution strategies, and training programs to the realities of clinical practice and patient preference.
Comparative regional analysis that explains how Americas, Europe Middle East & Africa, and Asia-Pacific differ in regulatory complexity, access pathways, and deployment strategies
Regional dynamics shape access models, regulatory engagement, and commercialization strategies, with each geographic cluster presenting distinct opportunities and constraints. The Americas encompass a wide spectrum of payer models and service delivery channels, where public health initiatives and private-sector innovation influence both uptake and outreach programs. Policy shifts and reimbursement structures in key countries drive procurement priorities and clinician practice patterns, while demographic trends and reproductive health policy debates shape patient demand and counseling needs.
Europe, Middle East & Africa present heterogeneous regulatory regimes and procurement models that require tailored registration strategies and region-specific evidence generation. In many subregions, strengthening supply chain security and building local clinical capacity are prerequisites to scaling access, while in others, established reimbursement frameworks and high clinician specialization support rapid deployment of new device technologies. Asia-Pacific displays rapid adoption potential driven by large population bases, increasing investment in reproductive health programs, and diverse channels that range from urban tertiary centers to community clinics. Regulatory harmonization efforts and regional manufacturing partnerships are particularly influential across this geography, affecting time-to-availability and commercial strategy. Across all regions, aligning regulatory engagement, clinician training, and distribution models to local health system realities remains a core determinant of successful adoption.
Competitive positioning and strategic plays that show how product innovation, evidence generation, and channel partnerships create sustainable advantage in the IUD sector
Competitive dynamics in the intrauterine device arena are driven by product differentiation, clinical evidence generation, and strategic channel partnerships. Leading manufacturers increasingly invest in next-generation materials science and delivery systems to reduce insertion complexity and broaden candidacy, while second-tier firms focus on targeted niches and cost-effective supply models. Strategic partnerships with distributors and specialty pharmacy channels enable broader geographic reach and improved inventory velocity, and alliances with clinical training organizations accelerate clinician adoption and minimize procedural complications.
R&D strategies emphasize post-market data collection and real-world evidence to demonstrate longevity, safety, and patient satisfaction, which supports positioning in clinical guidelines and payer conversations. Companies are also exploring modular service offerings-such as bundled training, insertion kits, and patient education toolkits-to differentiate beyond unit-level features. Supply-chain resiliency programs and regional manufacturing investments are common strategic responses to geopolitical and tariff pressures, with an emphasis on maintaining high quality standards while reducing lead times. Overall, competitive advantage increasingly depends on the ability to align device innovation with scalable service models, credible clinical evidence, and flexible commercial partnerships that address end-user needs across diverse care settings.
High-impact recommendations for leaders to combine product innovation, channel flexibility, and supply chain resilience to accelerate adoption and sustain advantage
Industry leaders should prioritize integrated strategies that combine product innovation with service design, supply chain resilience, and clinician enablement to capture growth while safeguarding quality. First, invest in device usability enhancements and real-world evidence programs that substantiate claims around safety, duration, and patient satisfaction, thereby reinforcing adoption among conservative clinical adopters and guideline committees. Next, expand distribution flexibility by fostering partnerships across hospital pharmacies, online pharmacies, and retail pharmacies to create multiple access points that align with patient preferences and institutional procurement models.
Operationally, strengthen supply chain resilience through regional manufacturing partnerships and diversified supplier networks to reduce exposure to tariff and logistical shocks. Build clinician training programs that are scalable and digitally enabled, lowering procedural variability and improving patient counseling outcomes. Finally, adopt outcome-oriented commercial models that bundle devices with training and follow-up services, making it easier for health systems to realize predictable clinical and operational benefits. By executing these priorities in a coordinated manner, organizations can accelerate adoption, protect margins, and enhance patient-centered care delivery.
Transparent mixed-methods research approach combining clinician interviews, regulatory review, and supply chain analysis to ensure actionable, validated insights
This research applied a mixed-methods approach that integrates primary stakeholder interviews, clinical literature synthesis, regulatory pathway reviews, and supply chain analysis to construct a robust evidence base. Primary engagements included structured interviews with clinicians, procurement professionals, and distribution executives to capture on-the-ground perspectives regarding device selection, procedural workflows, and inventory management. Clinical and regulatory sources were reviewed to validate safety profiles, labeling developments, and post-market surveillance trends that influence adoption and lifecycle decisions.
Quantitative inputs were contextualized through qualitative insights to ensure findings reflect operational realities, such as insertion training requirements and counseling workflows. Supply chain and trade-policy analyses incorporated publicly available customs and policy documents alongside expert interviews to trace impacts on sourcing decisions and logistical costs. Throughout, the methodology emphasized triangulation across data streams to minimize bias and to surface actionable insights relevant to commercial strategy, clinical adoption, and operational planning.
Concise synthesis of key strategic imperatives linking device innovation, evidence generation, and resilient operations to sustained clinical and commercial success
In conclusion, the intrauterine device landscape is characterized by converging pressures and opportunities that require coordinated strategic responses across product development, distribution, and service delivery. Technological advancements and patient-centered care models broaden the range of viable options for clinicians and patients, while policy and trade dynamics highlight the need for supply chain adaptability. Segmentation insights reveal that device choice, distribution pathways, clinical setting, and duration profiles jointly determine adoption patterns, underscoring the importance of aligning offerings to the operational realities of end users.
Companies that integrate robust evidence generation with clinician training, flexible distribution strategies, and resilient supply-chain planning will be best positioned to capture sustained clinical uptake and programmatic value. The path forward emphasizes pragmatic innovation, rigorous post-market evaluation, and close collaboration with healthcare providers to ensure devices translate into sustained patient benefit and efficient healthcare delivery.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Intrauterine Devices Market
Companies Mentioned
The key companies profiled in this Intrauterine Devices market report include:- AbbVie Inc.
- Bayer AG
- BioFem Therapeutics, LLC
- Chongqing Zhifei Biological Products Co., Ltd.
- CooperCompanies, Inc.
- Gedeon Richter Plc.
- Medicines360, Inc.
- Meril Life Sciences Pvt. Ltd.
- Mona Lisa N.V.
- Mylan NV by Viatris Inc.
- Novartis AG
- Ocon Medical Ltd
- Pregna International Ltd.
- Prosan International BV
- Searchlight Pharma Inc.
- Shenzhen Salubris Pharmaceuticals Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
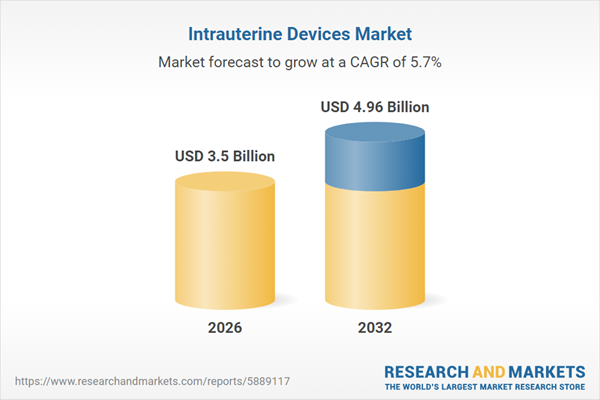
| Estimated Market Value ( USD | $ 3.5 Billion |
| Forecasted Market Value ( USD | $ 4.96 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 17 |