Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative introduction to intravascular warming systems that frames clinical utility, technological components, and implementation considerations for healthcare stakeholders
Intravascular warming systems have emerged as a critical adjunct to perioperative and critical care pathways, enabling clinicians to manage patient core temperature with greater precision than external methods alone. These systems integrate intravascular catheters and dedicated control units to deliver targeted warming or cooling through conductive and convective mechanisms, and their use has expanded across specialties due to demonstrated effects on hemodynamic stability, coagulation, and recovery trajectories. As hospitals and specialty clinics prioritize improved clinical outcomes and resource optimization, intravascular approaches are increasingly considered where rapid, controllable temperature modulation is required.Clinicians select intravascular warming solutions for scenarios in which surface warming is insufficient or impractical, including complex cardiac procedures, thermoregulatory failure, and postoperative temperature maintenance. Concurrently, technological advances in catheter design and control unit engineering have reduced procedural complexity and improved safety profiles, which fosters broader adoption. Regulatory scrutiny, reimbursement dynamics, and interoperability with hospital systems remain central considerations that influence procurement decisions and clinical pathway integration.
As a result, stakeholders across clinical, procurement, and engineering functions must assess device capabilities alongside institutional workflows. Transitioning from conceptual understanding to operational deployment requires coordinated training, protocol development, and alignment with quality and safety initiatives. By situating intravascular warming systems within the broader care continuum, healthcare providers can better evaluate clinical trade-offs and prioritize implementation strategies that align with patient populations and organizational objectives.
How converging clinical priorities, modular technology designs, and evolving procurement models are redefining adoption pathways and competitive dynamics in intravascular temperature management
The landscape for intravascular warming systems is being reshaped by converging clinical imperatives, technological innovation, and shifting procurement behaviors, producing transformative shifts in how these devices are developed, adopted, and scaled within health systems. Clinically, there is growing recognition of temperature management as an active therapeutic intervention rather than a passive supportive measure, which elevates demand for systems that deliver precise, responsive control and integrate with electronic medical records and perioperative workflows. In parallel, designers are moving beyond single-modality solutions toward platforms that offer modularity-interchangeable catheters and control units that support different heating methods-thus enabling institutions to tailor approaches to specific clinical scenarios.On the technology front, advances in materials science and miniaturization have refined catheter profiles and lumen architectures, improving ease of insertion and reducing patient risk. Control units are increasingly incorporating closed-loop feedback, refined resistive heating elements, and fluid circulation modes that allow clinicians to choose between gravity-fed simplicity and pump-driven precision. Concurrently, digitalization efforts are enabling remote monitoring and data capture for quality improvement initiatives, which in turn supports value-based care metrics and performance reporting. Supply chain and distribution models are also adapting: direct sales channels coexist with specialized distributors and growing online procurement pathways that accelerate access for smaller institutions and specialty clinics.
Taken together, these shifts are fostering a more competitive environment in which incumbents must demonstrate clinical evidence, operational compatibility, and economic value. As stakeholders navigate this evolving landscape, strategic partnerships between clinical innovators, control unit manufacturers, and catheter specialists are likely to intensify, while regulatory and reimbursement strategies will shape the timeline and scope of broader adoption.
Assessing the operational and strategic consequences of new tariff measures on cross-border sourcing, pricing strategies, and supply chain resilience for intravascular device stakeholders
The introduction of new tariff measures originating from the United States in 2025 has introduced a set of operational and strategic considerations for manufacturers, distributors, and healthcare providers engaged with intravascular warming systems. Increased cross-border costs can affect component sourcing decisions, prompting manufacturers to reassess supplier footprints and potentially re-shore or near-shore critical manufacturing steps to mitigate exposure. This reconfiguration can alter production lead times and capital expenditure planning, and therefore must be evaluated alongside clinical supply continuity and regulatory compliance obligations.Moreover, tariffs exert downstream effects on distribution economics, with import duties influencing pricing strategies adopted by distributors and direct sales teams. In response, contract terms between manufacturers and healthcare providers may evolve to include longer procurement cycles, hedging mechanisms, or clause adjustments that address tariff volatility. Hospitals and specialty clinics may also adapt procurement behavior by consolidating orders, prioritizing devices with stronger local manufacturing bases, or exploring alternative technologies that present lower import dependencies.
From a strategic perspective, the tariffs underscore the importance of supply chain resilience and diversification. Manufacturers can respond by qualifying multiple component suppliers across regions and by investing in manufacturing flexibility that enables rapid configuration changes. In addition, stronger collaboration between commercial teams and clinical stakeholders can help justify investments required to localize production through demonstrated clinical and operational benefits. Ultimately, while tariffs present an immediate cost and planning challenge, they also accelerate strategic initiatives that improve long-term supply security and operational agility.
High-resolution segmentation analysis that connects product architectures, clinical applications, end-user environments, and distribution pathways to strategic R&D and commercialization decisions
Understanding segmentation dynamics is essential to aligning product development, clinical positioning, and go-to-market strategies for intravascular warming systems. Based on product type, the market encompasses both catheter technologies and control units; catheters are differentiated by lumen configuration with single lumen designs offering simplified profiles and double lumen options enabling distinct circulation channels, while control units diverge between fluid circulation and resistive heating architectures, the former further subdividing into gravity-driven and pump-driven flow approaches and the latter incorporating PTC element and resistive wire heating methods that influence response time and thermal uniformity. These distinctions matter for clinicians who weigh insertion complexity, thermal transfer characteristics, and compatibility with existing vascular access practices.Meanwhile, application-based segmentation differentiates hypothermia management from temperature maintenance; within hypothermia management, devices are tailored to cardiac surgery cooling protocols and therapeutic cooling use cases, whereas temperature maintenance covers febrile treatment and postoperative recovery scenarios where steady-state control and patient comfort are priorities. Application context drives clinical requirements such as cooling or warming rate, monitoring granularity, and integration with adjunctive support devices, and thus informs product specification and clinical training needs.
End-user segmentation influences purchasing authority and deployment patterns, with hospitals representing primary adopters across emergency departments, intensive care units, and operating rooms, while specialty clinics pursue focused use cases that demand compact footprints and simplified interfaces. Technology segmentation further frames competitive positioning, as electrical resistance, fluid circulation, and magnetic induction technologies each offer trade-offs in terms of control fidelity, energy efficiency, and catheter complexity. Distribution channel segmentation-direct sales, distributors, and online-affects customer engagement and post-sale service models, with direct channels affording deeper clinical support and online pathways enabling scaled access for decentralized providers. Taken together, these segmentation lenses guide prioritization of R&D investments, clinical evidence generation, and commercialization tactics to match distinct stakeholder expectations.
A region-specific perspective on regulatory complexity, procurement behavior, and clinical adoption trends that informs localized commercialization and partnership strategies
Regional dynamics continue to shape adoption pathways and strategic priorities for intravascular warming systems across the Americas, Europe, Middle East & Africa, and Asia-Pacific, each presenting distinct regulatory regimes, reimbursement landscapes, and healthcare delivery models. In the Americas, advanced tertiary centers and established cardiac surgery programs drive demand for high-performance systems, while procurement practices emphasize evidence-based adoption, bundled purchasing, and the ability to support large surgical volumes. This environment incentivizes manufacturers to demonstrate clinical outcomes and to maintain robust service networks to support complex institution needs.Across Europe, the Middle East & Africa, purchasers balance centralized procurement frameworks and diverse national regulations, which places a premium on regulatory alignment and modular product offerings that can be tailored to varying clinical protocols. Reimbursement pathways and hospital budgeting cycles often influence adoption timing, and the presence of well-established critical care networks supports targeted clinical trials and guideline development. In the Asia-Pacific region, rapid expansion of tertiary care capacity and growing investments in minimally invasive technologies are accelerating interest in intravascular solutions; however, pricing sensitivity and heterogeneous regulatory regimes require flexible commercial models and strong local partnerships to achieve scale.
Taken together, these regional nuances necessitate adaptive strategies that combine rigorous clinical engagement with tailored distribution models. Manufacturers and distributors should prioritize regulatory readiness, localized training programs, and maintenance infrastructures that reflect the operational realities of each region, thereby ensuring consistent clinical performance and sustainable market access.
Competitive landscape appraisal emphasizing clinical partnerships, product modularity, and integration capabilities that determine supplier differentiation and long-term value propositions
Competitive dynamics in the intravascular warming systems space reflect a mix of established medical device manufacturers, specialized catheter innovators, and emerging technology entrants that emphasize digital integration and differentiated heating modalities. Leading players typically leverage broader cardiovascular portfolios, established clinical relationships, and global service networks to secure hospital contracts and support high-acuity use cases, while newer entrants focus on disruptive catheter designs, improved usability, or niche applications to gain traction in specialty clinics and select hospital units.Strategic activity is concentrated around product line extensions, interoperability initiatives, and targeted collaborations with clinical centers to generate real-world evidence. In addition, strategic investors and corporate development teams are increasingly evaluating opportunities to acquire complementary technologies that expand thermal control capabilities or enhance control unit software. This competitive landscape rewards companies that can demonstrate clear clinical benefits, simplified workflows, and robust post-market support.
Further, differentiation is increasingly driven by lifecycle considerations: modular systems that allow catheter interchangeability with a common control platform reduce customer switching costs and support recurring consumable revenue, whereas units that provide advanced monitoring analytics can be positioned as clinical quality enablers. For stakeholders evaluating partnerships or procurement options, assessing a supplier’s clinical data generation capacity, service infrastructure, and roadmap for integration with hospital IT systems is essential for long-term alignment.
Actionable strategic playbook for industry leaders focused on modular platforms, evidence generation, supply chain resilience, and hybrid commercial models to accelerate adoption
Industry leaders seeking to strengthen their position in the intravascular warming systems market should adopt a set of actionable measures that address product innovation, clinical validation, and commercial execution. First, prioritize modular platform strategies that enable a common control unit to support multiple catheter types and heating modalities; this reduces adoption barriers for hospitals and specialty clinics and enhances lifecycle revenue opportunities. Second, invest in clinician-focused evidence generation through multicenter observational initiatives and pragmatic clinical studies that highlight real-world benefits such as reduced complications, streamlined workflows, and improved recovery metrics. These data will bolster reimbursement conversations and procurement justifications.Third, strengthen supply chain resilience by qualifying geographically diverse suppliers and by evaluating localized manufacturing or final assembly options to mitigate tariff and trade disruptions. Fourth, enhance post-sale value through comprehensive training programs, digital remote monitoring capabilities, and service packages that minimize downtime and reinforce clinical confidence. Fifth, tailor commercial models to regional dynamics by combining direct clinical engagement in high-acuity centers with distributor-led and online channels for decentralized adoption; this hybrid approach accelerates market penetration while preserving high-touch support where it matters most.
Finally, pursue strategic partnerships with hospital systems and clinical opinion leaders to co-develop protocols and to position intravascular warming systems as integral components of broader perioperative and critical care pathways. By executing on these priorities in a coordinated manner, industry leaders can improve adoption velocity, demonstrate sustained clinical impact, and create defensible differentiation.
A transparent, multi-method research approach combining clinician interviews, device feature analysis, regulatory mapping, and supply chain scenario testing to validate findings
This research synthesizes technical, clinical, and commercial insights derived from a structured methodology that combines qualitative and quantitative techniques to ensure comprehensive coverage of the intravascular warming systems ecosystem. Primary inputs include interviews with clinicians across emergency departments, intensive care units, and operating rooms, discussions with device engineers and procurement professionals, and consultations with regulatory specialists to map approval pathways and post-market surveillance expectations. Secondary sources encompass peer-reviewed clinical literature, standards documents, and industry reports focused on device technology, clinical practice patterns, and supply chain considerations.Analytical approaches employed include comparative device feature analysis to evaluate catheter and control unit designs, technology readiness assessments across electrical resistance, fluid circulation, and magnetic induction approaches, and business model mapping to understand distribution and service implications. In addition, scenario-based supply chain stress testing was used to examine the potential operational impact of tariff and trade disruptions. Throughout the study, triangulation was applied to validate findings across multiple data sources, and subject-matter expert review ensured clinical relevance and methodological rigor.
Transparency measures included documentation of interview protocols, inclusion criteria for literature review, and a description of analytical assumptions where applicable. These elements together underpin a robust analytical foundation designed to inform strategic decision-making for manufacturers, healthcare providers, and distributors evaluating intravascular warming system opportunities.
Concluding synthesis emphasizing the interplay of clinical validation, modular product design, and supply chain resilience as the drivers of sustainable adoption in clinical practice
Intravascular warming systems occupy a strategic niche at the intersection of clinical efficacy, technological innovation, and operational pragmatism. Their ability to provide precise thermal control addresses unmet needs in cardiac surgery, therapeutic cooling, and postoperative temperature maintenance, and ongoing innovations in catheter architecture and control unit functionality are expanding clinical utility while simplifying integration into care pathways. Nevertheless, successful adoption hinges on clear clinical evidence, interoperable systems design, and resilient supply chains that can withstand geopolitical and trade-related pressures.Stakeholders should therefore adopt a balanced approach that couples aggressive clinical engagement with prudent operational planning. By prioritizing modular product development, investing in real-world evidence generation, and building flexible commercial and manufacturing models, manufacturers and healthcare providers can mitigate adoption barriers and demonstrate value to payers and procurement committees. Meanwhile, distributors and channel partners should align service offerings and training programs to support institutions across diverse settings, from high-volume tertiary centers to decentralized specialty clinics.
As healthcare systems continue to focus on quality outcomes and cost efficiency, intravascular warming systems that deliver measurable clinical benefits and operational reliability are well-positioned to become standard tools in temperature management. The path forward requires coordinated action across R&D, clinical affairs, and commercial teams to ensure that innovations translate into sustained improvements in patient care.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Intravascular Warming Systems Market
Companies Mentioned
The key companies profiled in this Intravascular Warming Systems market report include:- 3M Company
- Augustine Temperature Management LLC
- Barkey GmbH & Co. KG
- Becton, Dickinson and Company
- Belmont Instrument Corporation
- Biegler GmbH
- Enthermics Medical Systems, Inc.
- Geratherm Medical AG
- Hirtz & Co. KG
- Inditherm Plc
- Inspiration Healthcare Group plc
- Medtronic plc
- Smiths Medical Inc.
- Stihler Electronic GmbH
- Stryker Corporation
- The Surgical Company Group
- Vyaire Medical, Inc.
- ZOLL Medical Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
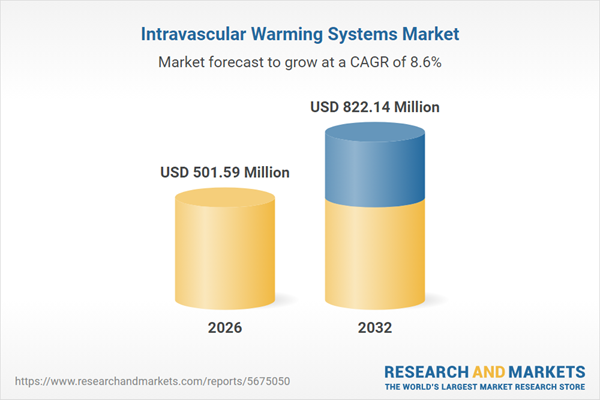
| Estimated Market Value ( USD | $ 501.59 Million |
| Forecasted Market Value ( USD | $ 822.14 Million |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |