Speak directly to the analyst to clarify any post sales queries you may have.
A strategic orientation to the evolving clinical, technological, and provider dynamics that define contemporary knee reconstruction decision-making and deployment
Knee reconstruction is at a pivotal juncture where clinical expectations, technology maturation, and health system priorities converge to reshape decision-making across providers and suppliers. Surgeons are balancing established surgical techniques with emerging guidance systems, while hospital administrators and ambulatory surgical center operators are weighing procedural throughput, equipment investments, and patient outcomes. At the same time, device developers and technology vendors are pushing toward solutions that promise greater precision, reproducibility, and alignment with value-based care initiatives.To navigate this complex environment, stakeholders need a concise synthesis of the principal drivers influencing clinical practice and procurement. This introduction synthesizes recent clinical adoption patterns, procedural pathway refinements, and the interplay of regulatory and reimbursement considerations that influence purchasing and deployment of knee reconstruction technologies. Taken together, these forces create opportunities for differentiated clinical value, while also imposing new expectations for training, interoperability, and perioperative workflows.
How technological advances, data-driven clinical workflows, and shifts in care delivery models are reshaping knee reconstruction practice and procurement decisions
The knee reconstruction landscape is undergoing transformative shifts driven by innovation in surgical assistance, data-driven perioperative management, and evolving care delivery models. Advanced navigation and robotics are moving beyond novelty toward integrated solutions that support reproducibility and patient-specific planning. Simultaneously, improvements in implant surface technologies and fixation techniques are influencing surgeon preference and long-term implant performance considerations.Furthermore, the rise of ambulatory settings and emphasis on faster recovery have pressured procedural pathways to become leaner and more standardized. Digital tools that enable preoperative templating, intraoperative guidance, and post-operative outcome tracking are becoming essential components of the clinical stack. These shifts are accompanied by intensified collaboration between device manufacturers, software developers, and health systems to ensure seamless adoption and to demonstrate real-world clinical and economic advantages across care settings.
Qualitative assessment of how recent tariff and trade dynamics are reconfiguring procurement, supply chain resilience, and partnership strategies in knee reconstruction
Policy shifts influencing tariffs and trade in 2025 have introduced new complexity into global procurement of surgical implants, instrumentation, and advanced guidance systems. Import duties and related compliance measures can increase landed costs for devices and peripherals, creating pressure on hospital budgets and procurement cycles. In response, supply chain leaders have had to reassess sourcing strategies, evaluate alternative distribution models, and strengthen inventory resilience to avoid procedural disruptions.Consequently, purchasers are placing greater emphasis on supplier partnerships that offer flexible logistics, local warehousing, and risk-sharing terms. Manufacturers and distributors are adapting by diversifying production footprints, negotiating tariff classification clarifications, and investing in regional supply bases to mitigate exposure to cross-border trade fluctuations. These adjustments have ripple effects across pricing negotiations, contract structures, and the timing of product introductions, and they also influence long-term decisions around capital equipment investments and service agreements.
Segment-driven strategic implications that connect technology choice, clinical setting, implant type, and fixation strategy to operational and procurement priorities across providers
Segment-level insights reveal differentiated adoption patterns and strategic priorities that providers and suppliers must recognize. Based on Technology, clinical teams and administrators are evaluating Computer Assisted Navigation and Robotics Assisted solutions for their potential to improve alignment and reproducibility while balancing the continued relevance of Conventional Manual approaches and the growing interest in Patient Specific Instrumentation for individualized fit. These technology choices cascade into training needs, capital planning, and intraoperative workflow design.Based on End User, Ambulatory Surgical Centers are prioritizing streamlined instrumentation and shorter turnover times to support higher throughput, whereas Hospitals often emphasize comprehensive service offerings, complex case capability, and integration with broader orthopedic programs. Based on Product Type, clinical decision-making varies between indications: decisions for Partial Knee Replacement and Patellofemoral Arthroplasty emphasize tissue preservation and targeted restoration of biomechanics, while Revision Knee Replacement and Total Knee Replacement require robust revision strategies, implant versatility, and contingency instrumentation. Based on Fixation, fixation strategy choices among Cemented, Cementless, and Hybrid implants influence instrument sets, postoperative protocols, and long-term outcome monitoring, and they affect inventory profiles and supplier contractual terms.
Regional commercialization and adoption dynamics that illuminate how reimbursement, regulatory diversity, and clinical infrastructure drive differentiated opportunities across global regions
Regional perspectives shape how technologies are commercialized, adopted, and supported. In the Americas, health systems and ambulatory centers demonstrate a varied pace of clinical adoption influenced by payer models, reimbursement policies, and a strong focus on patient-reported outcome measures that drive interest in advanced guidance and implant options. Suppliers competing in this region often emphasize local service capabilities, robust training programs, and partnerships that highlight value beyond the device.In Europe, Middle East & Africa, heterogeneous regulatory landscapes and diverse hospital capabilities create opportunities for tiered product strategies and flexible go-to-market approaches. Stakeholders in this region tend to prioritize clinical evidence, cost-effectiveness, and adaptability to varied care settings. In the Asia-Pacific region, rapid expansion of orthopedic infrastructure, investment in minimally invasive techniques, and growing capabilities in domestic manufacturing foster a dynamic environment for introducing both established implants and next-generation surgical assistance systems. Across all regions, customer support, training ecosystems, and supply chain responsiveness remain decisive factors for sustained adoption.
Competitive and collaborative dynamics that reveal how integrated solutions, aftermarket services, and targeted partnerships are shaping the evolving supplier landscape in knee reconstruction
Competitive dynamics in knee reconstruction are shaped by a blend of clinical innovation, strategic partnerships, and service-oriented differentiation. Leading industry participants are increasingly investing in integrated solutions that combine implants, instrumentation, digital planning, and intraoperative guidance to offer cohesive clinical value propositions. At the same time, specialized robotics and navigation firms focus on clinical evidence generation and surgeon training to accelerate acceptance and to secure procedural footholds in high-volume centers.Service and aftermarket support have become as strategic as the devices themselves, with warranty structures, instrumentation bundles, and consumable economics playing a central role in purchasing decisions. Collaborations between implant developers, technology vendors, and health systems are fostering bundled offerings that aim to reduce adoption friction. Meanwhile, contract manufacturers and regional distributors are expanding capabilities to support localization and responsive logistics, positioning themselves as essential partners in a fragmented and capital-intensive supply chain.
Practical and prioritized recommendations for developers, suppliers, and provider organizations to accelerate adoption, reduce friction, and align innovation with clinical value objectives
Leaders in the industry should pursue a set of pragmatic actions to align product development, commercial strategy, and clinical adoption. First, prioritize modular and interoperable technology architectures that enable clinical teams to adopt components progressively while protecting prior investments. This approach reduces adoption barriers and facilitates integration with existing surgical workflows. Second, strengthen clinical training and credentialing programs tied to measurable procedural competencies to accelerate safe, repeatable use of advanced guidance and robotic systems.Third, cultivate flexible commercial models that reflect the needs of both ambulatory surgical centers and hospitals, including bundled pricing, subscription-based access to software or analytics, and risk-sharing on outcomes where appropriate. Fourth, invest in local supply chain capabilities and post-market evidence generation to support regional regulatory requirements and procurement preferences. Finally, engage in transparent dialogue with payers, providers, and surgeon champions to align on value metrics and to build the real-world evidence that underpins longer-term adoption.
A rigorous mixed-methods research framework combining primary clinician engagement, secondary evidence synthesis, and iterative validation to ensure reliable and actionable findings
This study employs a mixed-method research approach that integrates primary stakeholder engagement with rigorous secondary analysis and structured validation. Primary inputs include qualitative interviews with orthopedic surgeons, supply chain leaders, and clinical administrators across ambulatory and hospital settings, designed to capture real-world operational constraints, clinical preferences, and procurement considerations. These interviews are augmented by device-specific technical appraisals and hands-on assessments of guidance systems, instrumentation sets, and fixation technologies.Secondary research incorporates publicly available regulatory filings, clinical literature, and policy documents to contextualize adoption drivers and safety considerations. Findings are triangulated through cross-validation with supplier product documentation and independent clinical advisories to ensure coherence and robustness. Finally, methodological transparency is maintained through documentation of interview protocols, inclusion criteria for secondary sources, and iterative peer review cycles that validate interpretations and thematic conclusions.
Concluding synthesis that distills how alignment across technology design, clinical training, and supply chain resilience will determine sustainable adoption and patient outcomes
In conclusion, knee reconstruction is experiencing a period of pragmatic innovation where clinical priorities, technological capability, and health system economics intersect to create selective opportunities for improved patient care. Key choices around technology adoption, implant selection, and fixation strategy must be made with an appreciation for the downstream operational and training implications, as well as the procurement and supply chain realities that govern device availability and total procedural cost of delivery.Moving forward, stakeholders who align product design with interoperability, invest in clinician training, and cultivate resilient supply chains will be best positioned to translate technological promise into consistent clinical outcomes. Collaborative approaches that link manufacturers, technology providers, and clinical leaders to generate meaningful evidence will remain central to widespread and sustainable adoption.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Knee Reconstruction Market
Companies Mentioned
The key companies profiled in this Knee Reconstruction market report include:- Arthrex, Inc.
- B. Braun Melsungen AG
- ConforMIS, Inc.
- Corin Group Limited
- DePuy Synthes, Inc.
- Exactech, Inc.
- MicroPort Scientific Corporation
- Smith & Nephew plc
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
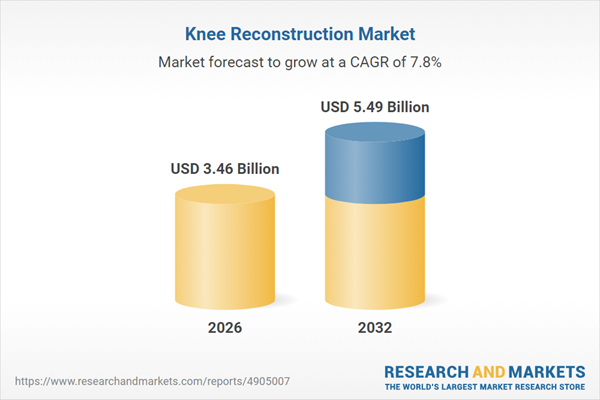
| Estimated Market Value ( USD | $ 3.46 Billion |
| Forecasted Market Value ( USD | $ 5.49 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |