Speak directly to the analyst to clarify any post sales queries you may have.
An expansive introduction to the laboratory chemicals sector that frames current market dynamics stakeholder priorities innovation trends and near term strategic imperatives
The laboratory chemicals sector stands at the intersection of rigorous scientific demand and complex commercial realities. Rising requirements for higher-purity reagents, expanding applications across diagnostics and research, and an intensifying focus on regulatory compliance are converging to redefine supplier priorities and customer expectations. In addition, the sector is responding to a renewed emphasis on sustainable sourcing and greener chemistries, which is reshaping procurement criteria for academic, clinical, and industrial end users. These forces are complemented by technological advances in analytical instrumentation and automation that increase throughput and raise quality thresholds for chemical inputs.
Consequently, laboratory chemical suppliers must balance legacy supply chain models with evolving performance metrics that prioritize traceability, reproducibility, and environmental impact. Stakeholders are increasingly attentive to lifecycle considerations, from raw material provenance through waste disposal, and this attention is translating into procurement requirements that extend beyond simple cost comparisons. As market participants adapt, strategic investments in quality assurance, certification pathways, and digital traceability will distinguish leaders from laggards. Ultimately, the introduction sets the stage for a deeper evaluation of how innovation, regulation, and shifting end-user needs will shape the competitive agenda and operational roadmaps across the sector.
A detailed examination of transformative shifts reshaping the laboratory chemicals ecosystem including technological advances sustainability mandates and supply chain reconfiguration
Significant shifts are underway that will reconfigure how laboratory chemicals are produced, distributed, and consumed. Digital transformation across procurement and quality systems is enabling near real-time inventory management and predictive replenishment, which reduces stockouts and improves laboratory efficiency. Parallel to this, advanced manufacturing techniques and modular synthesis capabilities are accelerating the pace at which specialized reagents and custom chemistries can reach end users, shrinking lead times and enabling more bespoke offerings.
Environmental regulations and corporate sustainability commitments are also driving meaningful change. Suppliers are reformulating products to reduce hazardous constituents, increasing the availability of greener alternatives, and adopting extended producer responsibility principles for chemical packaging. At the same time, geopolitical dynamics and trade policy shifts are incentivizing diversification of raw material sourcing and nearshoring strategies to insulate critical supplies. These dynamics are reinforced by evolving customer expectations for transparency around material provenance and compliance documentation. Taken together, technological, regulatory, and geopolitical forces are creating an environment where agility, traceability, and sustainability are table stakes for long-term competitiveness.
A comprehensive analysis of the cumulative impact of United States tariff changes in 2025 on laboratory chemical supply chains cost structures compliance burdens and market behavior
The 2025 changes in tariff policy introduced by the United States have produced a layered set of effects across laboratory chemical supply chains, commercial pricing, and compliance workloads. Increased duties on certain imported precursors and finished reagents have elevated landed costs for some categories, prompting purchasing teams to re-evaluate supplier networks and contract terms. In response, several organizations initiated near-term cost mitigation measures that included adjusting inventory buffers, negotiating longer-term fixed-price agreements, and accelerating qualification of domestic or alternative international suppliers.
Beyond immediate cost implications, the tariff environment has shifted strategic investments. Some suppliers and buyers have allocated additional resources to customs compliance, tariff classification, and origin documentation to optimize duty treatment and leverage available exemptions. Others have pursued vertical integration where feasible to reduce exposure to cross-border tariff volatility. Trade policy adjustments have also stimulated conversations around reshoring of critical chemical manufacturing and the establishment of regional supply nodes to shorten transit times and simplify regulatory interfaces. Importantly, the policy changes have not had uniform impact across all chemical segments; those dependent on highly specialized international intermediates face different trade-offs than suppliers of commodity-grade reagents. As a result, companies that proactively reassess procurement strategies, enhance supplier qualification processes, and invest in regulatory expertise are positioned to manage tariff-related disruption more effectively.
Insightful segmentation driven perspectives illuminating how chemical type form application end user purity grade and distribution channel interact to define strategic opportunity and risk
Understanding the market requires a granular view of segmentation across multiple dimensions, each of which drives distinct demand patterns and supplier strategies. Based on chemical type, the landscape divides into biochemical offerings, inorganic reagents, and organic compounds; biochemical materials encompass amino acids, enzymes, and proteins which are critical for diagnostics and bioprocessing workflows, while inorganic categories such as acids, bases, and salts underpin a broad set of analytical and formulation tasks, and organic classes including alcohols, hydrocarbons, and ketones are central to synthesis and solvent applications. In terms of form, products are supplied as gases, liquids, or powders, and each form factor creates unique handling, storage, and transportation considerations that influence distribution economics and safety protocols.
Application-wise, the market supports analytical testing, diagnostic workflows, pharmaceutical development, and research and development. Pharmaceutical use cases further segment into drug discovery, formulation, and process development, reflecting differing purity and documentation needs. Research and development applications span academic R&D, biotech R&D, and pharmaceutical R&D, each with varied purchasing cadence and customization requirements. End users comprise academic and government institutions, chemical companies, clinical and diagnostic laboratories, and pharmaceutical and biotechnology firms, all of which impose distinct service-level expectations and regulatory demands. Purity grade differentiation, from ACS and analytical through HPLC, reagent, and USP grades, fundamentally affects pricing, testing frequency, and shelf-life considerations. Finally, distribution channels include direct sales, distributors, and online platforms, which shape customer experience, lead times, and opportunities for value-added services. Integrating these segmentation lenses reveals where margin pools and growth opportunities align with technical capabilities and market needs, thereby informing product development and commercial strategies.
A regionally nuanced assessment of demand drivers regulatory regimes R and D hubs and supply resilience across the Americas Europe Middle East and Africa and Asia Pacific geographies
Regional dynamics exert a powerful influence on how laboratory chemicals are sourced, regulated, and consumed. In the Americas, a concentrated base of pharmaceutical manufacturing and academic research creates steady demand for high-purity reagents, alongside strong emphasis on regulatory compliance and traceability. North American supply chains are increasingly oriented toward resilience, with buyers pursuing multiple sourcing lanes and strategic partnerships to mitigate disruption risks. Meanwhile, Latin American markets demonstrate growing demand driven by expansion in diagnostics and localized manufacturing, yet they often contend with infrastructure and logistics constraints that affect lead times and cost-efficiency.
Across Europe, the Middle East & Africa, regulatory harmonization efforts and stringent environmental standards shape product formulations and packaging choices. European markets emphasize sustainability credentials, extended documentation, and circular economy considerations, while markets in the Middle East and Africa present opportunities tied to nascent clinical capacity expansion and industrial chemical demand. In the Asia-Pacific region, expansive manufacturing capacity, a deep supplier ecosystem, and rapid growth in biotech hubs underpin a robust supply base for both commodity and specialty chemicals. However, regulatory heterogeneity and varied quality assurance practices across jurisdictions require rigorous supplier qualification and localized compliance strategies. Collectively, these regional characteristics inform where companies should concentrate investments in production capacity, quality infrastructure, and localized commercial support to optimize service levels and capture strategic growth.
A forward looking synthesis of competitive behaviors partnership models and capability investments among leading laboratory chemical companies that influence innovation and distribution
Leading firms in the laboratory chemicals space are pursuing a mix of strategies to secure competitive advantage, including specialization in high-value chemistries, expansion of certified purity portfolios, and investments in digital customer engagement. Companies that emphasize traceability and provide extended documentation packages are winning greater trust among clinical and pharmaceutical customers who require validated supply chains and consistent quality. At the same time, strategic partnerships and targeted acquisitions remain a core playbook to acquire complementary capabilities such as custom synthesis, advanced packaging, or regional distribution networks that lower time-to-market for new offerings.
Operationally, successful companies are improving margins through lean manufacturing, enhanced analytical testing capabilities, and tighter quality control pathways that reduce batch variability and returns. On the commercial front, an increasing number of suppliers are deploying e-commerce platforms and integrated ordering systems to streamline replenishment for routine laboratory users, while offering specialized account management for complex institutional customers. Sustainability is becoming a differentiator as well, with market leaders pursuing greener product lines and end-of-life stewardship programs to meet customer requirements and regulatory expectations. Together, these company-level moves illustrate that a combined focus on technical excellence, distribution agility, and customer-centric services defines the profile of high-performing competitors.
Actionable recommendations tailored for industry leaders to enhance supply resilience regulatory compliance sustainability performance and commercial agility in laboratory chemicals
Industry leaders should pursue a multi-pronged strategy to enhance resilience, capture differentiated value, and meet evolving customer expectations. First, diversify sourcing strategies by qualifying secondary suppliers and developing regional supply nodes to reduce exposure to single-source risks and tariff vulnerabilities. Complement this approach with strengthened supplier qualification processes and enhanced material traceability to meet heightened compliance requirements. Second, prioritize investment in higher-purity product lines and expanded certification services that align with pharmaceutical and clinical laboratory needs, thereby creating defensible margins and deeper client relationships.
Third, accelerate digital adoption across order management, inventory forecasting, and quality documentation to reduce administrative friction and improve fulfillment speed. Fourth, embed sustainability into product design and packaging, including options for greener reagents and recyclable containment, which can be leveraged as a commercial differentiator in sensitive markets. Fifth, cultivate strategic collaborations with academic institutions, biotech firms, and instrumentation providers to co-develop tailored reagents and integrate chemical solutions into broader workflow offerings. Finally, enhance commercial models by combining direct sales for enterprise accounts with efficient distributor and online channels for transactional customers, enabling scalable coverage while preserving high-touch service where required. Implementing these recommendations will position organizations to navigate policy shifts, capture emerging demand, and build enduring competitive advantages.
A transparent and reproducible research methodology describing data sources primary research secondary validation expert consultations and limitations to ensure robust conclusions
The research underpinning this analysis employed a triangulated approach that integrates primary interviews, targeted data collection, and rigorous secondary-source validation. Primary research included structured discussions with procurement leaders, quality assurance managers, and R&D stakeholders across academic, clinical, and industrial organizations to capture firsthand perspectives on supplier performance, pain points, and procurement priorities. These qualitative inputs were complemented by consultations with industry experts on regulatory trends and supply chain configuration to contextualize observed commercial behaviors.
Secondary research drew on publicly available regulatory guidance, company disclosures, trade publications, and technical literature to map product categories, purity grade requirements, and distribution practices. Where possible, findings were cross-validated through multiple independent sources to reduce bias and enhance reliability. Analytical methods included thematic synthesis of qualitative data, comparative assessment of regional regulatory frameworks, and scenario analysis to explore the operational impact of tariff changes and supply disruptions. Limitations of the methodology include variability in publicly disclosed information across jurisdictions and the dynamic nature of policy developments; accordingly, readers are encouraged to engage directly for customized updates that reflect the most current local conditions.
A conclusive synthesis summarizing strategic implications for suppliers end users and policymakers emphasizing resilience differentiation and investment priorities for the laboratory chemicals sector
In summary, the laboratory chemicals sector is experiencing structural change driven by technological advances, regulatory pressures, and shifting trade policies. These forces are reshaping procurement preferences, elevating purity and traceability requirements, and creating new imperatives around sustainability and supply resilience. Companies that invest in high-quality manufacturing, digitalized operations, and targeted commercial channels will be better positioned to meet complex customer requirements and to withstand external shocks. Moreover, the segmentation of demand across chemical type, form, application, end user, and purity grade highlights the importance of differentiated product portfolios and adaptable distribution strategies.
Policymakers and industry stakeholders alike must recognize that maintaining robust access to essential laboratory inputs requires a combination of diversified sourcing, clear regulatory pathways, and collaborative approaches to sustainability. As organizations calibrate their short- and medium-term plans, the clear priorities are to secure critical supply lines, strengthen quality assurance capabilities, and align product development with the evolving needs of diagnostics, pharmaceutical development, and research communities. Ultimately, resilient, transparent, and customer-focused suppliers will capture the greatest long-term value as the industry transitions to higher standards of performance and sustainability.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Laboratory Chemicals Market
Companies Mentioned
The key companies profiled in this Laboratory Chemicals market report include:- Agilent Technologies, Inc.
- AppliChem GmbH
- Avantor, Inc.
- Beckman Coulter, Inc.
- Bio-Rad Laboratories, Inc.
- Carlo Erba Reagents
- Corning Incorporated
- Danaher Corporation
- Kimetsan Kimya Sanayi ve Ticaret A.Ş.
- Loba Chemie Pvt. Ltd.
- Lonza Group Ltd.
- Merck KGaA
- New England Biolabs, Inc.
- PerkinElmer, Inc.
- Promega Corporation
- Sartorius AG
- Shimadzu Corporation
- Sisco Research Laboratories
- TCI Chemicals
- Thermo Fisher Scientific Inc.
- Wako Pure Chemical Industries, Ltd.
- Waters Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
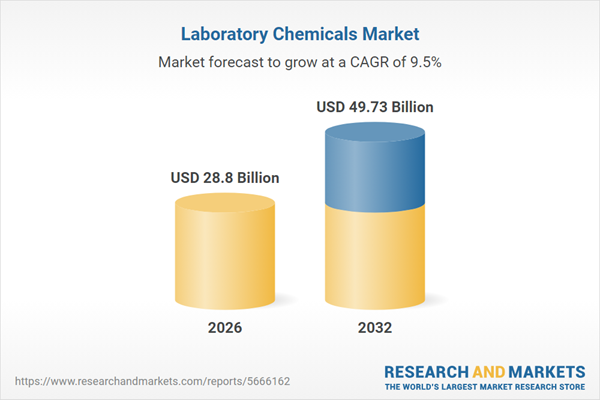
| Estimated Market Value ( USD | $ 28.8 Billion |
| Forecasted Market Value ( USD | $ 49.73 Billion |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |