Speak directly to the analyst to clarify any post sales queries you may have.
An executive introduction framing how advances in fiber, resin, manufacturing and clinical requirements are redefining strategic priorities across the medical composites value chain
The medical composites sector sits at the intersection of materials science, regulatory scrutiny, and clinical performance expectations. Advances in fiber technologies, resin chemistries, and manufacturing modalities are converging to expand the role of composites across device platforms, implants, and specialized clinical equipment. Clinicians and product developers increasingly value composites for their strength‑to‑weight advantages, design flexibility, and potential to meet stringent sterility and biocompatibility requirements.
Consequently, stakeholders across research and development, procurement, and regulatory affairs face a more complex decision landscape. Material selection must now integrate clinical performance data, lifecycle considerations, sterilization compatibility, and downstream manufacturing reproducibility. In addition, manufacturers are balancing the benefits of thermoplastic systems that enable faster processing and recyclability against the entrenched performance attributes of thermoset systems that have historically dominated high‑performance applications.
Moreover, the demand for customization - whether in patient‑specific implants or instrument components optimized for minimally invasive approaches - is prompting novel collaboration patterns between material suppliers, component manufacturers, and clinical research teams. These collaborations are catalyzing iteration cycles that combine additive manufacturing, prepreg layups, and traditional molding techniques to meet both functional and regulatory constraints. Overall, this introduction frames the broader forces that influence strategic choices, highlights the technical and operational trade‑offs organizations face, and sets the stage for the deeper analyses that follow.
Critical transformative shifts in technology, regulation, and supply systems that are reshaping design decisions, manufacturing strategies, and competitive differentiation in medical composites
The landscape for medical composites is undergoing transformative shifts driven by technology maturation, evolving clinical needs, and supply chain realignment. Lightweight construction and the demand for higher performance are accelerating adoption of carbon and high‑performance glass fibers in prosthetics, orthopedic devices, and precision surgical instruments. At the same time, the emergence of engineered thermoplastics is altering design and production workflows by enabling faster cycle times and enhanced recyclability, which matter increasingly to procurement and sustainability teams.
In parallel, additive manufacturing and hybrid manufacturing processes are unlocking new geometries and patient‑specific solutions that were previously impractical with conventional methods. These capabilities are encouraging device designers to rethink device architectures and consolidate multi‑component assemblies into single printed or molded structures, reducing assembly steps and potential failure points. Consequently, validation and quality assurance processes have adapted to encompass new material characterization protocols, process monitoring practices, and sterilization compatibility studies.
Regulatory dynamics and payer expectations are also shifting market incentives. Regulators are placing greater emphasis on material traceability, biocompatibility evidence, and post‑market surveillance, while health systems increasingly seek devices that combine durability with lower lifecycle costs. In response, manufacturers are prioritizing interoperability of data across design, manufacturing, and clinical performance datasets to accelerate submissions and post‑market evidence generation. Together, these shifts are redefining competitive advantage from purely material performance to integrated capabilities across design, production, and evidence management.
Analyzing how tariff adjustments have catalyzed strategic sourcing shifts, supplier localization, and procurement resilience actions that affect manufacturing and product development dynamics
Recent tariff developments in the United States have created pronounced effects across sourcing, supplier strategies, and cost management for firms that rely on imported fibers, resins, and intermediate composite components. Tariff adjustments have prompted procurement teams to reassess supplier footprints, accelerating engagements with domestic manufacturers and regional partners to mitigate exposure to trade policy volatility. As a result, firms are placing renewed emphasis on supplier qualification timelines, dual sourcing, and long‑term procurement contracts that provide greater visibility and protection against sudden cost escalation.
Moreover, tariffs have influenced the economics of certain resin systems and fiber types, incentivizing manufacturers to explore alternative chemistries and feedstocks that have lower exposure to affected tariffs. This adaptive behavior extends to material substitutions within validated design envelopes where clinically acceptable, and to investments in manufacturing efficiency that reduce the total cost per component. In parallel, strategic inventory positioning has become more prevalent, with firms balancing the carrying costs of buffer stock against the potential operational disruptions that arise from tariff‑induced supply interruptions.
Consequently, research and development roadmaps reflect a stronger focus on localized supply chains and process innovations that reduce dependence on high‑tariff inputs. There has also been an uptick in collaborative vendor agreements and joint development programs aimed at co‑investing in regional production capacity for critical fibers and resin precursors. Taken together, these adjustments illustrate how trade policy has catalyzed a recalibration of commercial and technical strategies to preserve supply continuity, control unit costs, and maintain regulatory compliance in medical applications.
Comprehensive segmentation insights linking end use industries, resin chemistries, fiber classifications, manufacturing methods, form factors, and product families to actionable R&D and sourcing choices
Segmentation insights reveal nuanced opportunity spaces across end use industries, resin chemistries, fiber architectures, manufacturing processes, form factors, and product types, each carrying distinct implications for product designers and business strategists. By end use industry, aerospace and defense requirements emphasize structural integrity and fatigue resistance with subsegments including fuselage components, interior components, and wing components; automotive applications prioritize body panels, chassis components, and interior components where crash energy management and weight reduction are paramount; healthcare applications concentrate on medical devices, orthopedic implants, and prosthetics where biocompatibility and sterilization performance guide material choices. Marine applications focus on deck structures and hulls with corrosion and moisture resistance constraints, sports and leisure demand tailored solutions for bicycles, golf clubs, and rackets where responsiveness and rider feel are critical, and wind energy emphasizes blades, hubs, and nacelles that require long fatigue life and large‑scale manufacturing capability.
Resin type divides into thermoplastic and thermoset pathways with further specialization that shapes processing and lifecycle attributes. Thermoplastic resins such as PEEK, polypropylene (PP), and PPS are notable for their reprocessability and rapid cycle capabilities. Within PEEK, distinctions arise between carbon fiber reinforced and unfilled grades that influence stiffness and machinability. Polypropylene differentiates into copolymer and homopolymer variants that affect impact resistance and thermal properties. PPS appears in glass filled and unfilled configurations, each balancing mechanical performance with thermal stability. Thermoset systems include epoxy, polyester, and vinyl ester chemistries; epoxy families such as bisphenol‑A, cycloaliphatic, and novolac offer varied cure kinetics and thermal performance, while polyester types including DCPD, isophthalic, and orthophthalic span different corrosion resistance and cost profiles. Vinyl ester formulations such as Atlac, Derakane, and Veova present distinct toughness and chemical resistance attributes relevant to harsh environments.
Fiber type segmentation separates aramid, carbon, and glass fibers with internal differentiation that affects toughness, stiffness, and cost. Aramid fibers split into meta‑aramid and para‑aramid grades that trade off thermal performance and tensile behavior. Carbon fibers differentiate by precursor and microstructure into PAN‑based, pitch‑based, and rayon‑based variants that drive modulus, conductivity, and price. Glass fiber classes such as C‑Glass, E‑Glass, and S‑Glass provide a spectrum of dielectric properties, strength, and affordability. Manufacturing processes cover autoclave molding, compression molding, filament winding, hand lay‑up, pultrusion, and resin transfer molding, each presenting unique throughput, capital, and quality control considerations that influence suitability for specific medical or industrial applications. Form factor segmentation spans pipes and tubes, profiles and other forms, rods and bars, and sheets and plates; within profiles and others, fabrics and nonwoven mats represent textile architectures used in varied lamination strategies. Product type distinctions encompass bulk molding compound, filament wound products, prepreg, pultruded profiles, and sheet molding compound. Bulk molding compound includes conductive and standard variants that affect electrical performance; filament wound products cover pipes, tubes, and pressure vessels that require precise winding control; prepreg divides into carbon and glass prepregs for high‑performance layups; pultruded profiles range across angles, channels, and I‑beams for structural applications; and sheet molding compound differentiates premium and standard formulations that align with surface finish and mechanical requirements.
Together, these segmentation layers inform targeted R&D investments, supplier selection, and manufacturing pathway decisions. They also help product teams prioritize validation protocols and regulatory evidence packages aligned with the specific material architectures and application environments they choose to pursue.
Key regional dynamics and strategic implications for manufacturing footprint, regulatory engagement, and clinical adoption across the Americas, Europe Middle East Africa, and Asia Pacific
Regional dynamics shape competitive strategy through differences in manufacturing capacity, regulatory regimes, supply chain maturity, and clinical adoption patterns. The Americas region is characterized by strong engineering capability, a robust supplier base for high‑value carbon fibers and advanced thermoplastics, and a regulatory environment that emphasizes rigorous clinical evidence and traceability. These attributes favor complex, high‑performance medical applications where integration with clinical trials and post‑market surveillance is critical. In contrast, Europe, Middle East & Africa combines diverse regulatory frameworks with pockets of advanced manufacturing clusters, particularly in Western and Northern Europe, where sustainability mandates and circularity initiatives influence material choices and end‑of‑life considerations. This region often leads in policy‑driven adoption of recyclable thermoplastic systems and regional collaborations that connect material innovation with industrial policy objectives.
Asia‑Pacific displays heterogeneity across markets but is notable for rapid manufacturing scale‑up, strong capabilities in mid‑to‑high performance glass fibers and growing investments in carbon fiber capacity. The region’s cost competitiveness and expanding supplier ecosystems make it a focal point for high‑volume component production and for manufacturers seeking nearshore alternatives in response to trade policy shifts. Importantly, clinical adoption curves and procurement models vary across countries in the region, necessitating differentiated regulatory strategies and local clinical partnerships to support device introductions.
Across all regions, cross‑border collaboration and strategic partnerships are increasingly common as firms seek to combine technical expertise, regulatory know‑how, and localized production to reduce time to clinic and improve supply resilience. Consequently, regional insights guide site selection for manufacturing investments, the design of regulatory submission strategies, and the formation of commercial partnerships that align with regional reimbursement and procurement dynamics.
Key attributes that define leading organizations in medical composites, including material innovation, validated manufacturing platforms, regulatory integration, and collaborative commercialization approaches
Leading organizations in the medical composites space demonstrate a blend of technical depth, manufacturing scale, and regulatory acumen that informs competitive positioning. Some firms focus on upstream material innovation, advancing fiber architectures and resin formulations to meet higher performance and sterilization requirements, while others concentrate on downstream system integration, supplying finished components and assemblies tailored to medical device OEMs. Strategic differentiation often emerges from investments in validated manufacturing platforms, quality management systems aligned with medical device standards, and cross‑functional teams that bridge materials science with clinical evidence generation.
In addition, collaborative models have become more prevalent, with material suppliers working closely with contract manufacturers and device developers to co‑design components and accelerate qualification pathways. This approach reduces development timelines and distributes risk across partners, particularly when navigating stringent biocompatibility testing and sterilization validations. Companies that succeed in this environment typically maintain robust supplier quality programs, transparent traceability practices, and proactive regulatory engagement to anticipate evolving requirements.
Finally, firms that integrate sustainability into product design and demonstrate quantifiable lifecycle benefits tend to gain greater traction with procurement teams at hospitals and large health systems. By embracing circular design principles, recyclability where clinically appropriate, and process efficiency that reduces waste, these organizations position themselves to meet both clinical performance expectations and broader institutional sustainability mandates.
Actionable recommendations for industry leaders to bolster supply resilience, modernize manufacturing, embed regulatory foresight, and harness strategic partnerships for accelerated commercialization
Industry leaders should prioritize a set of coordinated actions that preserve supply continuity, accelerate clinical translation, and enhance competitive differentiation. First, strengthen supplier diversification strategies by qualifying regional and domestic sources for critical fibers and resins while maintaining validated dual sourcing to reduce disruption risk. Second, invest in manufacturing process modernization through automation, in‑line process monitoring, and advanced curing technologies that improve yield, reproducibility, and throughput without compromising validation rigor.
Third, align product development roadmaps with regulatory and clinical evidence requirements early in the design phase. Building comprehensive material characterization and sterilization compatibility studies into initial development timelines reduces downstream surprises and shortens time to clinical adoption. Fourth, evaluate material substitutions and hybrid architectures that preserve clinical performance while reducing exposure to tariff and feedstock volatility. These substitutions should be trialed within controlled validation protocols to ensure safety and functionality remain uncompromised. Fifth, embed sustainability metrics into product and process design, focusing on recyclability and waste reduction where clinically acceptable, to address procurement preferences and institutional mandates.
Finally, cultivate strategic partnerships across the value chain, including material suppliers, contract manufacturers, and clinical research organizations, to co‑invest in scale‑up activities and evidence generation. Such partnerships can accelerate commercialization, distribute risk, and create pathways for continuous improvement in both product performance and manufacturing economics.
A rigorous, practitioner‑oriented research methodology combining stakeholder interviews, technical literature review, standards alignment, and cross‑validation procedures to ensure actionable and reliable insights
The research methodology underpinning this report integrates primary stakeholder engagement, technical literature synthesis, and cross‑functional validation to ensure robustness and relevance. Primary inputs were gathered through structured interviews with material scientists, manufacturing engineers, regulatory specialists, and procurement leaders to capture operational realities and decision criteria across the value chain. This practitioner perspective was augmented by detailed analysis of peer‑reviewed materials science literature, standards documentation, and publicly available regulatory guidance to align technical interpretations with current best practices.
Analytical procedures emphasized traceability and reproducibility: material and process characterizations were reviewed against standardized testing protocols, while manufacturing capability assessments drew upon facility certifications, production process descriptions, and quality system documentation where available. In addition, case study analyses were used to illustrate practical applications and to surface lessons learned from recent product introductions and scale‑up efforts.
Throughout the methodology, cross‑validation steps reconciled practitioner input with documented evidence and technical benchmarks to reduce bias and increase confidence in the conclusions. Transparency in data sourcing and a focus on decision‑relevant metrics guided the structuring of insights to support strategic and operational planning by stakeholders in medical device development and composite manufacturing.
A concise conclusion emphasizing integrated approaches that align material innovation, manufacturing validation, regulatory strategy, and partnership models to realize medical composites potential
In closing, medical composites occupy a pivotal role in the evolution of medical device design and manufacturing, delivering performance advantages while introducing new technical and regulatory considerations. The confluence of advanced fibers, evolving resin systems, and hybrid manufacturing methods offers opportunities to rethink product architectures and to achieve better clinical and economic outcomes. At the same time, trade policy dynamics, regional production differences, and heightened regulatory expectations require organizations to be deliberate in sourcing, validation, and evidence generation strategies.
Consequently, successful players will be those that combine material science excellence with disciplined manufacturing validation and proactive regulatory engagement. They will also be adept at forming strategic partnerships that align technical capability with clinical pathways and purchasing requirements. By prioritizing supply resilience, process modernization, and early alignment of clinical and regulatory evidence generation, firms can accelerate safe and reliable deployment of composite‑based medical devices and components. The path forward rests on an integrated approach that harmonizes innovation with practical considerations of production, compliance, and end‑user value.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Medical Composites Market
Companies Mentioned
The key companies profiled in this Medical Composites market report include:- 3M Company
- ACP Composites, Inc.
- Arkema S.A.
- Avient Corporation
- Celanese Corporation
- Composiflex Corporation
- Dentsply Sirona Inc.
- DuPont de Nemours, Inc.
- Evonik Industries AG
- Koninklijke DSM N.V.
- Mitsubishi Chemical Corporation
- Royal DSM N.V.
- Saudi Basic Industries Corporation
- SGL Carbon SE
- Solvay SA
- Teijin Limited
- Victrex plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
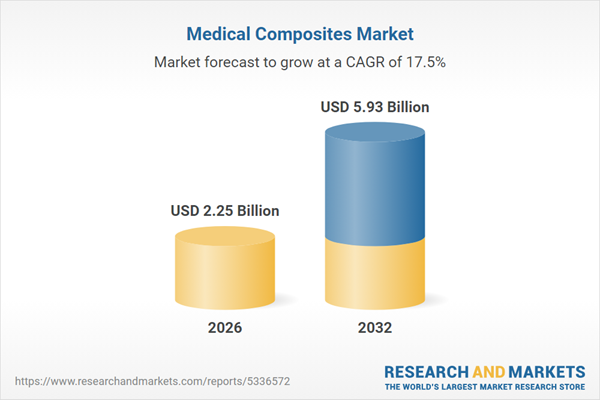
| Estimated Market Value ( USD | $ 2.25 Billion |
| Forecasted Market Value ( USD | $ 5.93 Billion |
| Compound Annual Growth Rate | 17.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |