Speak directly to the analyst to clarify any post sales queries you may have.
Unveiling the Strategic Imperatives and Historical Evolution of Mesalazine Therapy That Define Its Role in Modern Inflammatory Bowel Disease Management
Mesalazine, synthesized as an active moiety of sulfasalazine, emerged to address chronic inflammation in ulcerative colitis with its targeted anti-inflammatory mechanism. Originally formulated for oral administration with delayed-release coatings, it was designed to release 5-aminosalicylic acid in the distal ileum and colon, thereby mitigating systemic absorption and adverse effects. Over subsequent decades, innovator companies introduced controlled-release tablets, granules and delayed-release suspensions to tailor pharmacokinetics to patient needs and improve mucosal healing rates. In parallel, rectal enemas and suppositories were developed to directly target proctitis and distal colonic segments, expanding the therapeutic arsenal for localized disease manifestations. This evolution reflects a strategic commitment to balancing efficacy with tolerability, ensuring mesalazine remains a mainstay in both induction and maintenance phases of inflammatory bowel disease management.The clinical relevance of mesalazine extends beyond its anti-inflammatory properties. Extensive clinical trials and real-world studies have underscored its role in reducing relapse rates and corticosteroid dependency, reinforcing its favorable safety profile compared to systemic immunosuppressants. As a result, it has become integral to treatment guidelines worldwide, supported by consensus recommendations from leading gastroenterology societies. Looking ahead, ongoing research into biomarkers and patient stratification promises to refine mesalazine's positioning within personalized treatment paradigms, leveraging advances in molecular diagnostics to identify responders and optimize dosing strategies.
Additionally, collaborative research networks are harnessing real-world data registries to conduct post-market safety and effectiveness studies. These initiatives aim to refine mesalazine treatment algorithms by correlating patient demographics, genetic markers and longitudinal outcomes. Combined with innovations in patient-reported outcome measures, this approach is expected to shape future guidelines and optimize therapeutic pathways.
Examining the Disruptive Innovations Regulatory Amendments and Patient-Centric Trends Reshaping the Future Therapeutic Landscape of Mesalazine Use
The mesalazine landscape has experienced profound transformation driven by innovations in formulation science, regulatory evolution and a growing emphasis on patient-centric care models. Advanced drug delivery systems utilizing pH-sensitive coatings and multiparticulate granules have enhanced mucosal targeting, resulting in more consistent drug release along the intestinal tract. Meanwhile, the rise of digital health solutions such as electronic medication monitoring devices and mobile adherence apps has enabled real-time tracking of patient compliance, paving the way for integrated care pathways that prioritize long-term remission outcomes. These technological advances intersect with shifts in regulatory frameworks, as agencies increasingly require robust real-world evidence and post-marketing safety surveillance to ensure therapeutic benefit and tolerability.Concurrently, the competitive landscape is being reshaped by the entry of novel oral formulations and the introduction of generic alternatives with proprietary delivery platforms. Strategic alliances between biopharmaceutical companies and contract development organizations are accelerating development timelines for next-generation mesalazine therapies designed to improve bioavailability and reduce dosing frequency. In addition, emerging personalized medicine approaches that leverage genomic and microbiome profiling are driving research into targeted mesalazine regimens tailored to individual inflammatory signatures.
These converging trends herald a new era for mesalazine, where disruptive innovations and adaptive regulatory policies coalesce to foster optimized treatment paradigms. As the field progresses, stakeholders must navigate regulatory uncertainties and competitive pressures while capitalizing on technological breakthroughs to deliver differentiated therapeutic solutions and enhanced patient experiences.
In addition, the integration of telehealth consultations with pharmacist-led education programs is enhancing patient engagement and enabling proactive management of adherence challenges. Such service innovations are strengthening the overall care continuum for chronic inflammatory conditions.
Furthermore, environmental sustainability considerations are influencing manufacturing processes, prompting the adoption of green chemistry principles and waste reduction strategies in active pharmaceutical ingredient production. This growing focus on responsible sourcing and manufacturing resilience underscores the need for companies to integrate environmental, social and governance priorities into their strategic roadmaps.
Analyzing the Cascading Effects of United States Tariff Policies in 2025 on the Supply Chain Manufacturing Costs and Accessibility of Mesalazine Medications
The imposition of revised United States tariff measures in 2025 marks a pivotal inflection point for the mesalazine supply chain, with cascading ramifications for raw material sourcing, manufacturing economics and patient access. A substantial portion of the active pharmaceutical ingredient for mesalazine is produced in regions impacted by heightened import duties, leading manufacturers to reassess supplier contracts and reevaluate production locations. In response, some industry players are diversifying upstream partnerships to stabilize API availability, while others are investing in domestic capacity expansion to shield against cost volatility.These adjustments have translated into incremental increases in production expenditures, which in turn exert pressure on pricing negotiations within commercial and governmental procurement frameworks. Payers and health systems, facing constrained budgets, are demanding greater value demonstration and outcome-based contracting models to offset potential price upticks. As a result, manufacturers are exploring risk-sharing agreements and outcome-linked rebates to maintain formulary access and ensure continuity of care for patients reliant on mesalazine therapies.
Beyond direct cost impacts, the shift in tariff policy has intensified scrutiny of supply chain resilience, driving adoption of end-to-end visibility tools and advanced analytics to monitor logistics performance. This heightened focus on operational agility is prompting companies to establish strategic reserves and dual-source arrangements for critical intermediates. By proactively addressing tariff-induced uncertainties, stakeholders can mitigate disruptions, safeguard patient adherence and uphold the therapeutic momentum established by mesalazine in inflammatory bowel disease management.
Moreover, collaborative initiatives between manufacturers, distributors and healthcare providers are fostering enhanced transparency around inventory levels and anticipated demand, enabling more nimble response to market fluctuations. These collaborative frameworks are essential for sustaining uninterrupted access to mesalazine, particularly in regions with high prevalence of ulcerative colitis and associated inflammatory conditions.
Revealing In-Depth Product Form Administration Route Therapeutic Application End-User and Distribution Channel Perspectives for Precision Targeting
In-depth analysis across multiple segmentation dimensions reveals nuanced demand drivers and competitive positioning for mesalazine therapies. When assessing product form, tablets continue to account for widespread adoption owing to their convenience, although suppositories and rectal foam formulations are gaining traction for targeted treatment of distal colonic inflammation. Enemas and granules support tailored dosing regimens, particularly in patients exhibiting variable absorption kinetics. This diversification of dosage forms underscores the importance of aligning therapeutic strategies with anatomical disease localization and patient lifestyle considerations.Route of administration insights highlight a growing preference for oral delivery, driven by patient convenience and adherence benefits. However, rectal administration retains critical importance for localized disease management, supported by robust clinical evidence demonstrating rapid symptom relief in proctitis and left-sided colitis. Aligning formulation strategies with administration route preferences creates opportunities for differentiated value propositions and patient-centric support programs.
Application-based evaluation indicates that ulcerative colitis remains the predominant indication, yet mesalazine's role in Crohn's disease and broader inflammatory bowel disease contexts is expanding through off-label adoption and investigational protocols. Customizing dosing frameworks based on disease severity and anatomical involvement is essential for optimizing mucosal healing and minimizing systemic exposure.
End-user segmentation illustrates divergent utilization patterns across homecare settings and hospitals and clinics. Homecare markets are driven by self-administered therapies and patient education initiatives, whereas institutional settings prioritize hospital pharmacies for acute care administration. Distribution analysis reveals that hospital pharmacies, online pharmacies and retail pharmacies each fulfill distinct channels, with e-commerce platforms increasingly enabling direct-to-patient delivery and enhanced access in remote regions.
Payer incentives and reimbursement policies are increasingly reflecting segmentation nuances, with value-based contracts for suppositories in hospital settings and subscription models for self-administered tablet regimens, highlighting the intersection of clinical differentiation and economic models. Understanding these five interrelated segmentation axes empowers stakeholders to execute precision targeting, refine promotional tactics and elevate patient support services within the mesalazine ecosystem.
Interpreting Distinct Regional Demand Dynamics via the Americas Europe Middle East Africa and Asia Pacific Lenses to Optimize Market Outreach
Regional demand dynamics for mesalazine exhibit pronounced variations shaped by healthcare infrastructure maturity, reimbursement frameworks and disease prevalence patterns. In the Americas, robust clinical guideline endorsement and expansive insurance coverage facilitate broad access to established oral formulations, while emerging digital adherence tools are gaining momentum within patient support programs. The region's emphasis on value-based contracting incentivizes manufacturers to demonstrate real-world outcomes and cost-effectiveness through longitudinal studies and integrated care models.Moving to Europe, Middle East and Africa, heterogeneous regulatory landscapes and diverse payer ecosystems drive a patchwork of formulary access scenarios. Western European markets maintain high utilization of advanced delayed-release technologies and value added services, supported by stringent pharmacovigilance requirements. In contrast, emerging economies within the Middle East and Africa prioritize cost containment, leading to increased reliance on generic formulations and targeted distribution through hospital pharmacies and government tenders. This dynamic underscores the necessity for tailored market entry strategies and collaborative partnerships with local stakeholders to navigate regional reimbursement complexities.
In the Asia-Pacific region, accelerating urbanization and rising incidence of inflammatory bowel disease are catalyzing demand for innovative mesalazine therapies. Several markets are witnessing rapid expansion of retail and online pharmacy channels, complemented by government initiatives to improve chronic disease management. The growing middle class is influencing uptake of premium formulations, while regional manufacturers are driving cost competitiveness through localized production. Stakeholders must account for cultural preferences, regulatory heterogeneity and evolving digital ecosystems to capitalize on the substantial growth opportunities emerging across the Asia-Pacific landscape.
Spotlighting Leading Biopharmaceutical Innovators Strategic Collaborations and Pipeline Developments Driving Competitive Leadership in Mesalazine Solutions
Leading biopharmaceutical companies are actively shaping the mesalazine environment through strategic product portfolios, research collaborations and lifecycle management initiatives. Established players have fortified their positions with proprietary delivery platforms that extend drug release windows and reduce dosing frequency. Simultaneously, generic manufacturers are leveraging advanced formulation patents to introduce differentiated versions with unique release profiles, intensifying competitive dynamics.Collaborative alliances between innovator firms and contract development organizations have accelerated pipeline progression for novel mesalazine analogues and combination therapies, signaling a shift towards more sophisticated anti-inflammatory regimens. These partnerships harness specialized expertise in formulation science, regulatory navigation and clinical trial execution, expediting time to market and regulatory approvals in key jurisdictions.
Furthermore, strategic acquisitions and licensing agreements are reshaping company footprints, as larger entities integrate nimble biotech innovators to expand therapeutic arsenals. This consolidation trend is complemented by joint ventures aimed at optimizing supply chain efficiencies and broadening geographic reach, particularly in emerging markets. Companies investing in digital health ecosystems and patient support platforms are gaining differentiation, as data-driven insights into adherence patterns and real-world outcomes inform value communication strategies.
In addition, several organizations are exploring next-generation formulations that incorporate gut microbiome-modulating excipients and responsive release mechanisms. These cutting-edge research efforts could redefine treatment paradigms by aligning drug delivery with mucosal healing processes and minimizing systemic exposure.
Delivering Strategic Imperatives and Practical Guidelines for Industry Stakeholders to Enhance Patient Outcomes and Streamline Mesalazine Distribution
For industry leaders seeking to fortify their market positions in the mesalazine domain, several strategic imperatives emerge from current dynamics. Prioritizing investment in next-generation delivery platforms can differentiate product offerings and address unmet needs in patient adherence and localized inflammation control. By integrating smart packaging technologies with patient engagement solutions, companies can enhance medication compliance and capture valuable real-world evidence to support outcome-based contracting frameworks.Supply chain diversification is equally essential. Establishing multiple API sourcing agreements and regional manufacturing sites mitigates exposure to tariff fluctuations and geopolitical disruptions, preserving cost stability and ensuring uninterrupted therapeutic supply. Collaborative partnerships with logistics providers and technology firms can further optimize inventory management and distribution efficiency.
Engaging payers and healthcare providers through data-driven value dossiers and real-world performance metrics will facilitate broader formulary access and reimbursement alignment. Industry stakeholders should also explore risk-sharing and rebate models that align commercial terms with patient health outcomes, reinforcing the value proposition of mesalazine therapies.
Finally, companies should embrace a patient-centric mindset by deploying digital platforms for adherence monitoring, education and support services. These integrated solutions not only improve clinical outcomes but also generate longitudinal data that can inform strategic decision making, ultimately strengthening competitive advantage in the evolving mesalazine ecosystem.
Outlining Rigorous Multimodal Data Collection Analytical Frameworks and Validation Processes Underpinning the Comprehensive Study of Mesalazine Market Dynamics
This analysis was underpinned by a rigorous research methodology combining primary qualitative insights with comprehensive secondary data reviews. In the primary phase, in-depth interviews were conducted with gastroenterology specialists, hospital pharmacists and key opinion leaders across major regions to capture nuanced perspectives on clinical practice patterns, formulation preferences and emerging therapeutic trends. These discussions were complemented by detailed consultations with procurement and supply chain professionals to understand the operational impact of tariff shifts and distribution challenges.The secondary research component encompassed systematic examination of scientific literature, regulatory filings and company disclosures to collate information on formulation advancements, pipeline developments and strategic partnerships. Data triangulation techniques were applied to reconcile findings from various sources, ensuring consistency and validity. Quantitative market indicators such as regional usage statistics and prescription trends were analyzed alongside qualitative insights to provide a holistic view of demand drivers.
To validate the findings, interim reports were subjected to peer review by an external panel of academic researchers and industry consultants. Any discrepancies identified during the validation process were meticulously resolved through follow-up inquiries and data verification. Ethical compliance and data integrity protocols were strictly upheld throughout the research lifecycle, ensuring that insights presented reflect an accurate and unbiased portrayal of the mesalazine landscape.
Consolidating Strategic Insights and Forward-Looking Perspectives to Empower Stakeholders in Maximizing Therapeutic Impact with Mesalazine
The comprehensive exploration of mesalazine's evolution, market dynamics and strategic imperatives highlights its enduring significance in managing inflammatory bowel disease. Advancements in formulation technologies, digital adherence solutions and personalized treatment approaches are redefining the therapeutic landscape, while regulatory shifts and tariff policies underscore the importance of supply chain resilience. Segmentation and regional analyses reveal targeted opportunities for precision deployment, and corporate strategies centered on innovation collaborations and patient engagement are driving competitive differentiation.By synthesizing these insights, stakeholders can make informed decisions to optimize product positioning, navigate reimbursement complexities and enhance operational agility. As the field progresses towards more tailored therapeutic regimens and outcome-based care models, mesalazine remains central to delivering effective, patient-focused treatment. Embracing the strategic recommendations outlined herein will empower organizations to sustain leadership and improve patient outcomes in an increasingly complex and dynamic environment.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Form
- Enemas
- Granules
- Rectal Foam
- Suppositories
- Tablets
- Route Of Administration
- Oral
- Rectal
- Application
- Crohn's Disease
- Inflammatory Bowel Disease
- Ulcerative colitis
- End-User
- Homecare Settings
- Hospitals & Clinics
- Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AbbVie Inc.
- Allergan Pharmaceuticals International, Ltd.
- Amneal Pharmaceuticals, Inc.
- Apotex Inc.
- Axplora Group GmbH
- Cambrex Corporation
- Chemi S.p.A.
- CordenPharma International
- Divi's Laboratories Limited
- Dr. Falk Pharma GmbH
- Ethypharm SAS
- Ferring Pharmaceuticals
- Fresenius Kabi
- LGM Pharma
- Lupin limited
- Pfizer Inc.
- Rosemont Pharmaceuticals Ltd.
- Salix Pharmaceuticals, Inc.
- Sun Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceuticals
- Tillotts Pharma Ltd.
- Viatris Inc.
- Zhejiang Hengkang Pharmaceutical Co. Ltd.
- Zydus Pharmaceuticals, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Mesalazine market report include:- AbbVie Inc.
- Allergan Pharmaceuticals International, Ltd.
- Amneal Pharmaceuticals, Inc.
- Apotex Inc.
- Axplora Group GmbH
- Cambrex Corporation
- Chemi S.p.A.
- CordenPharma International
- Divi’s Laboratories Limited
- Dr. Falk Pharma GmbH
- Ethypharm SAS
- Ferring Pharmaceuticals
- Fresenius Kabi
- LGM Pharma
- Lupin limited
- Pfizer Inc.
- Rosemont Pharmaceuticals Ltd.
- Salix Pharmaceuticals, Inc.
- Sun Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceuticals
- Tillotts Pharma Ltd.
- Viatris Inc.
- Zhejiang Hengkang Pharmaceutical Co. Ltd.
- Zydus Pharmaceuticals, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
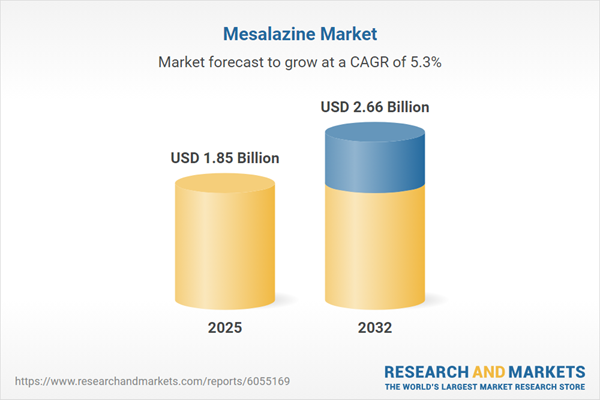
| Estimated Market Value ( USD | $ 1.85 Billion |
| Forecasted Market Value ( USD | $ 2.66 Billion |
| Compound Annual Growth Rate | 5.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |