Hospitals play a crucial role in the Automated External Defibrillator (AED) market by serving as key hubs for the deployment, utilization, and maintenance of these life-saving devices. Within hospital settings, AEDs are strategically placed in various locations such as emergency departments, intensive care units, and general wards to ensure rapid access during sudden cardiac arrest (SCA) emergencies. Therefore, the UAE market consumed 27.6 hundred units in Hospitals in the market in 2022.
The Brazil market dominated the LAMEA Automated External Defibrillator Market by Country in 2022 and would continue to be a dominant market till 2030; thereby, achieving a market value of $56.8 million by 2030. The Argentina market is showcasing a CAGR of 13% during (2023 - 2030). Additionally, The UAE market would register a CAGR of 12% during (2023 - 2030).
AEDs are strategically placed in educational institutions to ensure rapid response in case of sudden cardiac arrest. AEDs are present in community centers, ensuring emergency readiness during events and gatherings. Churches, mosques, synagogues, and other places of worship deploy AEDs for the safety of congregants. Buses, trains, and other public transport vehicles may be equipped with AEDs to respond to emergencies during transit.
A growing trend is the adoption of AEDs in homes, especially for individuals with known cardiac issues or those living with elderly family members. Having an AED at home can provide immediate assistance while waiting for professional medical help to arrive. Integration with smart technologies, such as Bluetooth connectivity and mobile applications, enhances AED functionality. Smart AEDs may provide real-time monitoring, improving overall effectiveness. AI integration in AEDs allows for a more precise analysis of cardiac rhythms.
As per the National Library of Medicine, the Middle East has a high rate of cardiovascular disease (10.1%). In this region, the prevalence of dyslipidemia (43.3%) is double that of hypertension (26.2%) and diabetes mellitus (16%). The UAE hosts numerous high-profile sporting events, attracting athletes, spectators, and visitors from everywhere. Some sports venues in the UAE may integrate AEDs with telehealth platforms to enable remote assistance. Thus, due to these aspects, the automated external defibrillator market will expand across the LAMEA region in upcoming years.
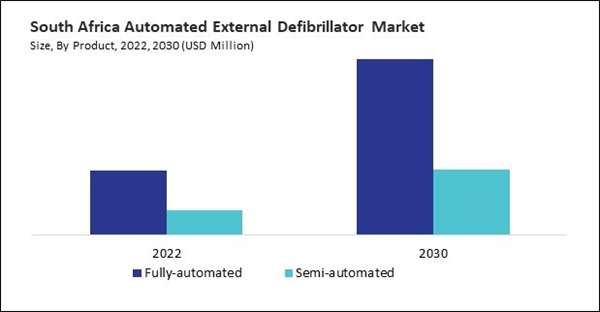
Based on Product, the market is segmented into Fully-automated, and Semi-automated. Based on End-use, the market is segmented into Hospital, Pre Hospital, Public Access, Homecare, and Others. Based on countries, the market is segmented into Brazil, Argentina, UAE, Saudi Arabia, South Africa, Nigeria, and Rest of LAMEA.
List of Key Companies Profiled
- Nihon Kohden Corporation (Defibtech LLC)
- Koninklijke Philips N.V.
- Bpl Medical Technologies Private Limited
- Corpuls (Nordic Capital)
- Progetti SRL
- Stryker Corporation (HeartSine Technologies, Ltd.)
- CU Medical Systems, Inc.
- Silverline Meditech Pvt. Ltd.
- AMI Italia S.r.l.
- Medtronic PLC
Market Report Segmentation
By Product (Volume, Hundred Units, USD Billion, 2019-2030)- Fully-automated
- Semi-automated
- Hospital
- Pre Hospital
- Public Access
- Homecare
- Others
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Table of Contents
Companies Mentioned
- Nihon Kohden Corporation (Defibtech LLC)
- Koninklijke Philips N.V.
- Bpl Medical Technologies Private Limited
- Corpuls (Nordic Capital)
- Progetti SRL
- Stryker Corporation (HeartSine Technologies, Ltd.)
- CU Medical Systems, Inc.
- Silverline Meditech Pvt. Ltd.
- AMI Italia S.r.l.
- Medtronic PLC