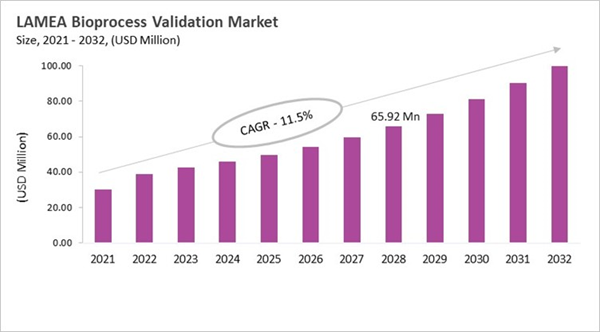
The Brazil market dominated the LAMEA Bioprocess Validation Market by country in 2024, and is expected to continue to be a dominant market till 2032; thereby, achieving a market value of USD 31.2 million by 2032. The Argentina market is showcasing a CAGR of 11% during 2025-2032. Additionally, the UAE market would register a CAGR of 9.4% during 2025-2032. The Brazil and UAE led the LAMEA Bioprocess Validation Market by Country with a market share of 33.8% and 14.6% in 2024.
The LAMEA bioprocess validation market is moving away from just following basic GMP rules and toward practices that are based on the whole lifecycle and are in line with international standards. Investments in biopharmaceutical infrastructure in Brazil, Mexico, South Africa, and the Middle East have led to a need for strict validation to meet global standards. Regional regulators like ANVISA, COFEPRIS, SAHPRA, and agencies in the Gulf states are working together with WHO and ICH guidelines. This makes people expect more continuous monitoring, risk-based approaches, and data integrity. More and more new greenfield plants are using single-use and modular systems. These systems lower capital costs, but they need to be thoroughly tested for extractables, leachables, and environmental robustness. Outsourcing is growing because smaller biotech companies and new businesses depend on global OEMs, multinational validation providers, or specialized local firms to quickly meet international audit requirements.
Top companies are coming up with plans that combine their global knowledge with their local presence. They set up regional validation labs and service hubs to speed up the process and build trust with regulators. They also work with local testing labs and universities to share knowledge and increase capacity. Modular validation kits and pre-validated assemblies make it easier for cost-sensitive markets to get in, and digital tools like predictive modeling cut down on long empirical testing. Risk-based validation proposals help balance the need to follow the rules with the need to stay within budget. Competition is getting tougher between multinational companies that offer bundled hardware and service packages and flexible regional providers that know the area well. Niche experts in areas like viral clearance and extractables are finding their place, and new technologies like automation and real-time monitoring are becoming important factors in setting companies apart, along with reputation and regulatory credibility.
Testing Type Outlook
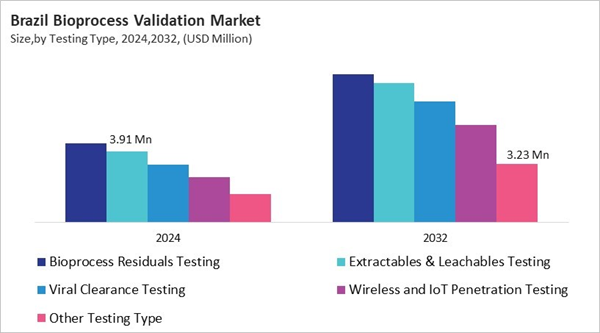
Based on Testing Type, the market is segmented into Bioprocess Residuals Testing, Extractables & Leachables Testing, Viral Clearance Testing, Wireless and IoT Penetration Testing, and Other Testing Type. Among various Brazil Bioprocess Validation Market by Testing Type; The Bioprocess Residuals Testing market achieved a market size of USD $4.4 Million in 2024 and is expected to grow at a CAGR of 8.4 % during the forecast period. The Wireless and IoT Penetration Testing market is predicted to experience a CAGR of 10.3% throughout the forecast period from (2025 - 2032).Mode Outlook
Based on Mode, the market is segmented into In house and Outsourced. The In house market segment dominated the UAE Bioprocess Validation Market by Mode is expected to grow at a CAGR of 9 % during the forecast period thereby continuing its dominance until 2032. Also, the Outsourced market is anticipated to grow as a CAGR of 10.7 % during the forecast period during 2025-2032.Country Outlook
Brazil's bioprocess validation market is growing quickly because the pharmaceutical and biotechnology sectors are strong and ANVISA keeps a close eye on things. As the demand for vaccines, biosimilars, and monoclonal antibodies grows, so does the need for thorough validation to make sure the products are safe and work. The market is growing even more because of rising healthcare costs, more chronic and infectious diseases, and government programs to boost biopharmaceutical production in the US. Companies are using automation, IoT-enabled monitoring, and digital platforms to keep track of quality in real time, do predictive analytics, and check processes all the time. Testing for extractables and leachables is now standard to make products safer. There are both domestic and international competitors. Local labs are improving their skills, and global companies are teaming up with Brazilian manufacturers to share technology and know-how. This combination makes Brazil a major center for advanced bioprocess validation in Latin America.List of Key Companies Profiled
- Merck KGaA
- Thermo Fisher Scientific, Inc.
- SGS S.A.
- Eurofins Scientific SE
- Sartorius AG
- Charles River Laboratories International, Inc.
- Lonza Group Ltd.
- WuXi AppTec Co., Ltd.
- Danaher Corporation
- Cobetter Filtration equipment Co., Ltd.
Market Report Segmentation
By Mode
- In house
- Outsourced
By Stage
- Continued Process Verification
- Process Qualification
- Process Design
By Testing Type
- Bioprocess Residuals Testing
- Extractables & Leachables Testing
- Viral Clearance Testing
- Wireless and IoT Penetration Testing
- Other Testing Type
By Country
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Table of Contents
Companies Mentioned
- Merck KGaA
- Thermo Fisher Scientific, Inc.
- SGS S.A.
- Eurofins Scientific SE
- Sartorius AG
- Charles River Laboratories International, Inc.
- Lonza Group Ltd.
- WuXi AppTec Co., Ltd.
- Danaher Corporation
- Cobetter Filtration equipment Co., Ltd.