Speak directly to the analyst to clarify any post sales queries you may have.
Unveiling the Multifaceted Evolution of Motion Sickness Treatment Strategies with Emphasis on Patient-Centric Innovations and Clinical Practice Dynamics
Motion sickness remains a pervasive challenge affecting travelers, sea voyagers, pilots, and even individuals using virtual reality applications. Characterized by nausea, dizziness, and disorientation, this condition undermines comfort and productivity across multiple environments. Historically, therapeutic interventions centered on general antiemetic approaches, yet recent years have witnessed a shift toward more targeted, patient-centric strategies that address underlying neurological and vestibular triggers. Clinicians now emphasize personalized regimens that balance efficacy with tolerability.With increasing understanding of neurotransmitter pathways and receptor interactions, innovations have emerged ranging from refined antihistaminic compounds to novel anticholinergic agents. Concurrently, nonpharmacological solutions have gained traction, encompassing behavioral training and external sensory modulation techniques. These developments occur against the backdrop of heightened patient expectations for rapid relief, minimal side effects, and accessible delivery formats.
This executive summary offers a holistic overview of the current motion sickness treatment landscape. It outlines the transformative shifts reshaping R&D, examines the effects of evolving tariff policies on supply chain dynamics, and presents critical segmentation and regional insights. By synthesizing key company strategies, actionable recommendations, and the rigorous methodology underpinning these findings, this summary aims to equip decision-makers with the clarity and foresight necessary to navigate an intricate and rapidly advancing therapeutic domain.
Exploring Transformative Shifts in Motion Sickness Therapeutic Landscape That Highlight Breakthrough Mechanistic Insights Regulatory Advancements and Evolving Adoption Trends
Recent breakthroughs in pharmacological research have redefined how motion sickness is managed across clinical and consumer settings. Researchers have drilled into novel receptor targets, identifying compounds that more precisely modulate vestibular pathways while minimizing central nervous system side effects. At the same time, regulatory bodies have accelerated review timelines for therapies demonstrating compelling safety profiles, expediting market entry for high-impact candidates.In parallel, digital health solutions now offer remote monitoring and dosage adjustment capabilities that adapt to individual physiological responses in real time. Wearable sensors and smartphone platforms allow for continuous tracking of motion-induced symptoms, empowering patients to engage proactively with their treatment plans. This convergence of pharmacotherapy and digital innovation marks a pivotal shift from one-size-fits-all approaches toward dynamic, adaptive care models.
Moreover, collaborations between academic institutions, device manufacturers, and pharmaceutical firms have established new frameworks for co-development, pooling expertise to streamline clinical validation of integrated treatment models. As a result, stakeholders are redefining outcome measures to incorporate patient-reported experience metrics alongside traditional efficacy endpoints, underscoring a more holistic vision of treatment success.
Examining the Cumulative Impact of United States Tariff Policies on Motion Sickness Treatment Supply Chains Clinical Costs and Strategic Sourcing Decisions through 2025
United States tariff policies implemented through 2025 have introduced significant variables for manufacturers and distributors of motion sickness treatments. Import duties on active pharmaceutical ingredients and specialized excipients have increased production costs, prompting companies to reassess procurement strategies and consider alternative sourcing locations. As a direct consequence, some key ingredients have shifted from established overseas suppliers to domestic or nearshore partners, aiming to mitigate exposure to tariff fluctuations.Simultaneously, these policy changes have incentivized investments in manufacturing infrastructure within the United States, thereby enhancing local capacity but also requiring capital-intensive facility expansions. Companies that have diversified their supply chains effectively have been better positioned to absorb incremental cost pressures and maintain stable inventory levels. However, smaller enterprises without flexible sourcing networks have encountered challenges in preserving margin structures under elevated duty regimes.
Looking ahead, strategic leaders are monitoring anticipated adjustments to trade agreements and exploring free trade zone opportunities to alleviate tariff burdens. By proactively recalibrating logistics routes and contractual terms, industry players are seeking to uphold affordability for end users while sustaining innovation pipelines in motion sickness therapeutics.
Deriving Key Insights from Segmentation Analyses of Motion Sickness Treatment across Drug Types Treatment Modalities Dosage Forms Distribution Channels and End User Environments
Insights derived from segmentation analyses reveal the nuanced preferences and performance dynamics of various therapeutic categories. When categorized by type of drugs, treatments leveraging anticholinergic agents have demonstrated robust efficacy in attenuating vestibular signals, while antihistaminic options continue to benefit from established safety records and widespread accessibility. In terms of treatment modality, patients increasingly weigh the appeal of natural remedies against the convenience of over-the-counter medications and the potency of prescription options, often weighing side effect profiles and onset of relief criteria.Exploring dosage form distribution underscores that oral formulations dominate volume-driven contexts thanks to ease of administration, whereas injectable solutions serve acute care scenarios in clinical settings and transdermal patches are gaining traction for steady-state delivery over extended travel durations. Within distribution channels, the growth of online pharmacies complements the extensive footprint of brick-and-mortar retail pharmacies, affording patients the flexibility to choose between home delivery and immediate in-store fulfillment.
End-user environments further shape product differentiation: clinics and hospitals demand rigorous compliance documentation and customizable dosing regimens, whereas home care contexts drive preferences for minimally invasive formats and user-friendly packaging. By overlaying these layers of segmentation, stakeholders can identify high-value niches and tailor development strategies to address specific patient cohorts and channel requirements.
Uncovering Pivotal Regional Dynamics Shaping Motion Sickness Treatment Adoption Patterns and Infrastructure Variations across Americas Europe Middle East Africa and Asia Pacific
Regional dynamics exert a profound influence on how motion sickness treatments are accessed, prescribed, and consumed. In the Americas, widespread awareness of travel-related motion discomfort has fostered innovation in consumer-oriented formulations and digital adherence tools, supported by well-established regulatory pathways and extensive pharmacy networks. This region's infrastructure facilitates rapid distribution of both traditional antihistamines and next-generation mechanistic therapies.Across Europe, the Middle East, and Africa, regulatory harmonization initiatives are smoothing market entry for novel agents, although the diversity of reimbursement environments and healthcare delivery models calls for localized commercialization strategies. In some markets, public healthcare systems prioritize cost containment, whereas in others, private payers emphasize premium products with demonstrable quality-of-life benefits.
Meanwhile, in the Asia-Pacific region, escalating tourism and burgeoning middle-class demographics have spurred demand for advanced motion sickness solutions. Manufacturers are optimizing product portfolios to align with regional preferences for natural ingredients and portable delivery systems, while forging partnerships with local distributors to navigate complex regulatory frameworks. Collectively, these territorial variations highlight the importance of adaptable business models and culturally informed messaging to maximize market penetration.
Highlighting Leading Innovation and Strategic Positioning by Key Pharmaceutical Companies Driving Development and Commercialization of Motion Sickness Treatment Solutions in a Competitive Ecosystem
Leading pharmaceutical and biotechnology firms are shaping the competitive landscape of motion sickness treatment through targeted R&D investments and strategic alliances. Several major players have enriched their pipelines with next-generation compounds that blend enhanced receptor specificity with favorable tolerability profiles. At the same time, cross-sector collaborations are enabling device integration within transdermal and wearable systems, bringing new modalities to market.Manufacturers are also pursuing licensing agreements to broaden geographic reach, securing distribution partners that offer deep local market insights. Innovative contract manufacturing organizations have emerged as critical allies, delivering scalable production solutions that support both early-stage clinical batches and commercial-scale output. As a result, companies with vertically integrated capabilities from discovery through distribution enjoy streamlined pathways to market and sustained operational resilience.
Furthermore, a growing emphasis on post-market surveillance and real-world evidence generation is reinforcing product narratives, enabling differentiation in a crowded field. By collecting patient-reported outcomes and adherence analytics, these industry leaders refine clinical support services and align their offerings with evolving payer criteria, cementing their positions at the forefront of motion sickness therapeutic innovation.
Formulating Actionable Recommendations for Industry Leaders to Capitalize on Emerging Motion Sickness Treatment Breakthroughs Optimize Supply Chains and Strengthen Patient Engagement Strategies
Industry leaders should prioritize the integration of personalized digital platforms that monitor vestibular responses in real time and adjust dosing regimens dynamically, thereby enhancing therapeutic precision and patient adherence. In addition, investing in combination formulations that pair receptor-targeted drugs with complementary nonpharmacological modalities can deliver multifaceted relief and reduce the risk of adverse effects.Moreover, companies must refine their supply chain architectures by establishing regional manufacturing hubs and diversifying supplier portfolios to buffer against tariff volatility. Collaborative agreements with logistics providers can optimize last-mile delivery and maintain continuity of care during peak travel seasons. Simultaneously, building robust pharmacovigilance frameworks that leverage patient-reported data will strengthen regulatory engagement and bolster reimbursement negotiations.
Finally, forging educational partnerships with healthcare practitioners and patient advocacy groups will amplify awareness of emerging treatments and foster trust. By delivering tailored training materials and in-field support, industry leaders can elevate clinical adoption rates and carve out distinctive value propositions. These actionable steps collectively position organizations to capitalize on the evolving motion sickness treatment landscape and deliver meaningful improvements in patient outcomes.
Detailing Rigorous Research Methodology Employed in Assessing Motion Sickness Treatment Landscape Including Data Collection Procedures Analytical Frameworks and Validation Protocols
This analysis synthesizes insights gleaned from a rigorous, multi-phased research process. Initially, subject-matter experts conducted in-depth interviews with key opinion leaders in neurology, otolaryngology, and travel medicine to capture frontline perspectives on therapeutic efficacy and patient experience. Concurrently, a comprehensive review of peer-reviewed journals, clinical trial registries, and patent filings provided empirical validation of emerging mechanistic pathways.Quantitative data were then aggregated from public healthcare databases and industry publications to elucidate distribution patterns, pricing structures, and regional adoption metrics. To enhance reliability, these findings underwent triangulation through secondary consultations with supply chain specialists and regulatory affairs professionals. Analytical frameworks incorporated both qualitative thematic coding and statistical trend analysis to ensure a balanced interpretation of market dynamics.
Finally, each insight was subjected to an internal validation protocol, where cross-functional teams assessed the consistency of conclusions against real-world case studies and proprietary product performance data. This methodical approach guarantees that the conclusions and recommendations presented herein rest on a foundation of robust evidence and expert consensus.
Concluding Strategic Perspectives on Motion Sickness Treatment Innovations Market Dynamics and Future Trajectories to Inform Decision Makers and Stakeholders Aligned with Emerging Healthcare Needs
In summary, the motion sickness treatment arena is undergoing a significant transformation fueled by targeted pharmacological advancements, regulatory evolutions, and innovative delivery mechanisms. Evolving tariff policies have highlighted the strategic importance of resilient supply chains, while detailed segmentation analyses underscore the need for customized solutions tailored to diverse patient groups and distribution channels.Regional insights reveal distinct market entry considerations, from infrastructure maturity in the Americas to regulatory diversity across Europe, the Middle East, and Africa, and burgeoning consumer demand in the Asia-Pacific zone. Meanwhile, key companies are fortifying their positions through pipeline expansion, collaborative partnerships, and enhanced patient support services.
By embracing digital integration, optimizing manufacturing footprints, and engaging stakeholders across the healthcare continuum, organizations can navigate the complexities of this dynamic field. The actionable strategies outlined here provide a roadmap for capturing growth opportunities and delivering enhanced quality of care for individuals affected by motion sickness.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Type Of Drugs
- Anticholinergics
- Antihistamines
- Treatment Type
- Natural Remedies
- Over-the-Counter Medications
- Prescription Medications
- Dosage Forms
- Injectable
- Oral
- Transdermal Patches
- Distribution Channel
- Online Pharmacies
- Retail Pharmacies
- End User
- Clinics
- Home Care
- Hospitals
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Amneal Pharmaceuticals LLC
- Astellas Pharma Inc.
- Baxter International Inc.,
- Bayer AG
- BONINE by Wellspring Pharmaceutical Corporation
- Cipla Limited
- CVS Health Corporation
- GlaxoSmithKline plc
- Lupin Limited
- Myungmoon Pharma Co. Ltd.
- Novartis AG
- Perrigo Company PLC
- Pfizer Inc.
- Prestige Consumer Healthcare Inc.,
- Reliefband Technologies LLC
- Sanofi S.A.
- Taj Pharmaceuticals Limited
- Teva Pharmaceutical Industries Limited
- Torrent Pharmaceuticals Ltd
- WellSpring Pharmaceutical Corporation
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Motion Sickness Treatment market report include:- Amneal Pharmaceuticals LLC
- Astellas Pharma Inc.
- Baxter International Inc.,
- Bayer AG
- BONINE by Wellspring Pharmaceutical Corporation
- Cipla Limited
- CVS Health Corporation
- GlaxoSmithKline plc
- Lupin Limited
- Myungmoon Pharma Co. Ltd.
- Novartis AG
- Perrigo Company PLC
- Pfizer Inc.
- Prestige Consumer Healthcare Inc.,
- Reliefband Technologies LLC
- Sanofi S.A.
- Taj Pharmaceuticals Limited
- Teva Pharmaceutical Industries Limited
- Torrent Pharmaceuticals Ltd
- WellSpring Pharmaceutical Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
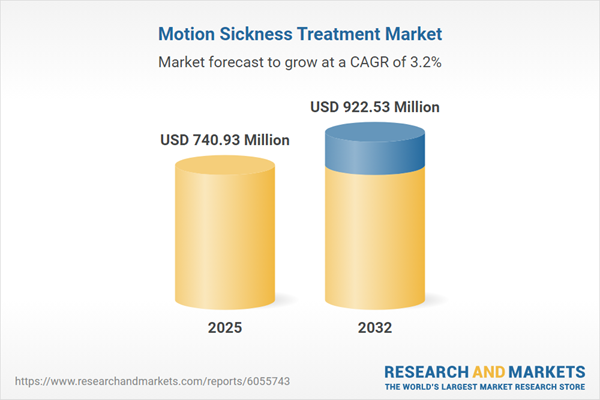
| Estimated Market Value ( USD | $ 740.93 Million |
| Forecasted Market Value ( USD | $ 922.53 Million |
| Compound Annual Growth Rate | 3.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |