Global MRI Compatible Pacemaker Market - Key Trends & Drivers Summarized
Why Are MRI Compatible Pacemakers Revolutionizing Cardiac Care?
MRI compatible pacemakers have become a crucial innovation in cardiac care, addressing a long-standing challenge faced by patients with traditional pacemakers. Historically, patients with standard pacemakers could not safely undergo magnetic resonance imaging (MRI) scans due to the risk of device malfunction or interference caused by the powerful magnetic fields. This posed a significant limitation, as MRIs are a critical diagnostic tool for various conditions, particularly for elderly patients who are more likely to require both pacemakers and MRI scans. The development of MRI compatible pacemakers has resolved this issue by incorporating materials and designs that allow patients to safely undergo full-body MRIs without compromising the pacemaker's functionality. This advancement is enabling better long-term health management for cardiac patients and improving their quality of life.How Is Technology Enhancing the Functionality of MRI Compatible Pacemakers?
Technological innovation is a key driver in the development of MRI compatible pacemakers, ensuring that these devices meet the growing demand for safe diagnostic imaging. Manufacturers are integrating advanced sensors, programmable features, and enhanced battery life into these devices to improve both their durability and performance in MRI environments. Key innovations include the use of non-magnetic materials, such as titanium and specific polymers, and the redesign of circuitry that can withstand the magnetic fields generated during MRI scans. These improvements not only enhance patient safety during MRI procedures but also contribute to the overall reliability and functionality of pacemakers in regular cardiac therapy. Additionally, remote monitoring capabilities in modern pacemakers allow physicians to track the patient's heart health in real-time, providing a comprehensive solution for continuous cardiac care.What Are the Regulatory and Market Trends Shaping the Adoption of MRI Compatible Pacemakers?
The growing adoption of MRI compatible pacemakers is being supported by favorable regulatory approvals and expanding healthcare infrastructure. In key markets like the United States and Europe, regulatory bodies such as the FDA and the European Medicines Agency have established clear guidelines for the approval and use of MRI compatible devices, ensuring their safety and effectiveness. As a result, the number of pacemaker manufacturers offering MRI compatible models is rising, creating increased competition and more affordable options for healthcare providers and patients alike. Emerging economies in Asia and Latin America are also experiencing increased demand for these devices due to the rising prevalence of cardiovascular diseases and improving access to advanced healthcare technologies. In parallel, demographic trends, particularly the aging global population, are further fueling the demand for cardiac care solutions, including MRI compatible pacemakers.The Growth in the MRI Compatible Pacemaker Market Is Driven by Several Factors
The growth in the MRI compatible pacemaker market is driven by several factors, including the increasing prevalence of cardiovascular diseases, especially among aging populations who are more likely to require pacemaker implants and frequent diagnostic imaging. Technological advancements, such as the development of more durable, programmable, and miniaturized devices, are also boosting adoption rates. Additionally, rising awareness among healthcare providers about the importance of MRI compatibility for comprehensive diagnostic care is leading to higher recommendations for these devices. Patient preferences for safer and more effective long-term cardiac solutions are further influencing the market, along with growing healthcare expenditure in both developed and emerging economies. Finally, regulatory support and the expansion of healthcare infrastructure are facilitating easier access to MRI compatible pacemakers, making them more widely available across global markets.Report Scope
The report analyzes the MRI Compatible Pacemaker market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Application (Arrhythmias, Congestive Heart Failure, Other Applications); End-Use (Hospitals & Cardiac Centers, Ambulatory Surgery Centers, Other End-Uses).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Arrhythmias Application segment, which is expected to reach US$1.5 Billion by 2030 with a CAGR of a 4.5%. The Congestive Heart Failure Application segment is also set to grow at 3.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $622.4 Million in 2024, and China, forecasted to grow at an impressive 6.3% CAGR to reach $607.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global MRI Compatible Pacemaker Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global MRI Compatible Pacemaker Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global MRI Compatible Pacemaker Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Biotronik SE & Co. KG, Boston Scientific Corporation, Lepu Medical Technology (Beijing) Co., Ltd., LivaNova PLC, Medico SpA and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 46 companies featured in this MRI Compatible Pacemaker market report include:
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Lepu Medical Technology (Beijing) Co., Ltd.
- LivaNova PLC
- Medico SpA
- Medtronic PLC
- Oscor, Inc.
- Osypka Medical GmbH
- Shree Pacetronix Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Lepu Medical Technology (Beijing) Co., Ltd.
- LivaNova PLC
- Medico SpA
- Medtronic PLC
- Oscor, Inc.
- Osypka Medical GmbH
- Shree Pacetronix Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
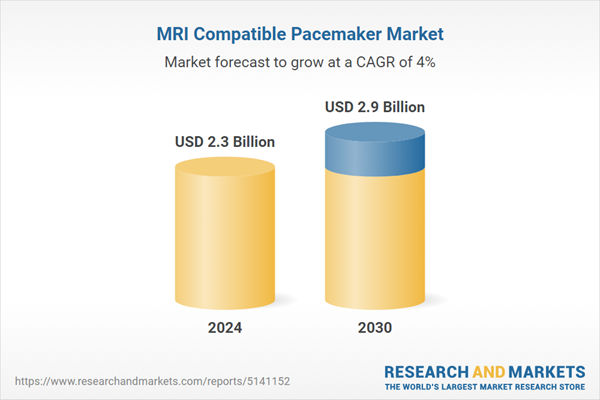
| Estimated Market Value ( USD | $ 2.3 Billion |
| Forecasted Market Value ( USD | $ 2.9 Billion |
| Compound Annual Growth Rate | 4.0% |
| Regions Covered | Global |