Speak directly to the analyst to clarify any post sales queries you may have.
A concise orientation to the clinical, technological, and regulatory forces reshaping nasal implant practice and device selection dynamics
Nasal implants occupy a critical intersection of reconstructive technique, implantable materials science, and evolving patient expectations. The convergence of advances in biomaterials, minimally invasive surgical approaches, and more stringent safety and regulatory scrutiny has reframed how clinicians and device developers approach product selection, procedure planning, and long-term performance evaluation. As a result, stakeholders from surgeons and hospital procurement teams to device manufacturers are recalibrating priorities around durability, biocompatibility, ease of handling, and post-operative outcomes.
Clinical trends emphasize patient-centric outcomes, with aesthetic refinement and functional restoration increasingly being assessed through validated patient-reported outcome measures. Simultaneously, technology-driven design improvements-ranging from anatomically contoured silicone shapes to porous expanded polytetrafluoroethylene scaffolds and refined approaches to autologous tissue grafting-are broadening clinical options. These shifts are occurring against a backdrop of supply chain complexity and shifting reimbursement environments, which together influence adoption curves and clinical practice patterns.
Looking ahead, integration of cross-disciplinary evidence, iterative clinical feedback, and real-world performance data will shape device evolution. This demands that industry actors maintain close collaboration with surgical opinion leaders, adapt to evolving regulatory expectations, and prioritize rigorous post-market surveillance to sustain clinician trust and patient safety.
How material innovation, procedural refinements, regulatory pressure, and value-based procurement are jointly accelerating product differentiation in nasal implants
The landscape for nasal implants is experiencing transformative shifts driven by innovation in materials, procedural techniques, and stakeholder expectations. On the materials frontier, there is a clear movement toward solutions that balance structural support with minimized inflammatory response; this preference is prompting renewed interest in autologous tissue approaches alongside optimized synthetic scaffolds designed for lower foreign-body reaction. Concurrently, surgeons are adopting less invasive delivery techniques and refined fixation strategies that reduce operative time and improve early recovery trajectories.
Procurement and distribution models are also evolving. Health systems are seeking greater transparency around cost drivers, total procedure cost, and device lifecycle considerations, encouraging manufacturers to offer value-based product propositions that address both clinical performance and operational efficiency. Regulatory frameworks are tightening in many jurisdictions, increasing the emphasis on long-term safety data and more comprehensive post-market surveillance programs. These shifts are amplifying the importance of clinical evidence generation, iterative design validation, and cross-functional collaboration between R&D, clinical affairs, and quality assurance teams.
As a result, companies that align product development with demonstrable clinical benefits and operational value are better positioned to capture clinician preference and institutional adoption. In short, the market is moving from commodity-oriented purchasing to clinical-value-driven selection, and that change is accelerating product differentiation on the basis of outcomes, ease of use, and safety transparency.
Scenario analysis of how tariff-driven input cost changes and supply chain adjustments could reshape sourcing, manufacturing and procurement decisions through 2025
Policy actions that affect import duties and trade conditions can have outsized effects on implantable device supply chains, procurement costs, and manufacturer sourcing strategies. When tariff measures are proposed or implemented, their cumulative impact typically manifests across several interrelated dimensions: raw material sourcing costs, manufacturing location economics, supply chain resiliency decisions, and downstream procurement practices among healthcare providers. In an environment where tariffs are a material factor for 2025 planning horizons, stakeholders must evaluate both the immediate cost pressures and the strategic responses that mitigate those pressures.
First, material-dependent product lines-such as silicone components or expanded polytetrafluoroethylene-are vulnerable to input cost changes that can reduce margin unless manufacturers absorb costs or pass them through. Second, tariffs can incentivize reshoring or nearshoring of manufacturing capacity, prompting capital allocation decisions and potential reconfiguration of supplier relationships. Third, healthcare purchasers may respond by placing greater emphasis on total cost of ownership and supplier diversification, which can alter contract structures and tender timelines.
Moreover, the cumulative effect of tariff-related frictions tends to accelerate adoption of alternative materials or techniques when such substitutions are clinically viable and cost-effective. Consequently, device developers and providers should use scenario planning to assess impacts on sourcing, pricing, product design, and contractual terms, while investing in flexible manufacturing and validated alternate-material pathways to preserve supply continuity and competitive positioning.
An integrated segmentation perspective showing how application, material choices, end-user settings, procedural distinctions and distribution strategies determine adoption and product priorities
A segmentation-focused view reveals how clinical, material, end-user, procedural, and distribution factors interact to shape adoption and product requirements. Based on application, clinicians differentiate products by their intended purpose, distinguishing cosmetic indications focused on aesthetic contour and soft-tissue support from functional indications aimed at restoring airway patency and structural integrity. This application split informs device design priorities and clinical evidence needs. Based on material type, implant technologies span autologous tissue, expanded polytetrafluoroethylene, and silicone, where autologous tissue is further categorized by bone graft and cartilage approaches; each material pathway carries distinct handling characteristics, biocompatibility profiles, and clinical trade-offs that influence surgeon preference and perioperative protocols.
Based on end user, adoption dynamics vary across ambulatory surgical centers, hospitals, and specialty clinics, with each channel exhibiting different purchasing processes, clinical staffing models, and reimbursement considerations that affect product selection. Based on procedure type, the distinction between primary and revision procedures drives divergent performance criteria-primary cases emphasize initial fit and predictability, while revision cases demand improved tissue integration and strategies to manage scarred anatomy. Based on distribution channel, manufacturers balance direct sales relationships against distributor networks to optimize market coverage and clinical support, recognizing that direct engagement often enables tighter clinical training programs while distributors can provide broader geographic reach and logistical support.
Together, these segmentation lenses create a nuanced matrix of clinical requirements, commercial strategies, and product development priorities that manufacturers and providers must reconcile to achieve successful market penetration and sustained clinician adoption.
How regional regulatory diversity, clinical practice norms, and manufacturing capabilities across major geographies influence adoption patterns and commercialization strategies
Regional dynamics exert a material influence on regulatory pathways, clinician preferences, supply chain logistics, and payer environments. In the Americas, practice patterns emphasize a mix of aesthetic and reconstructive indications with an established ecosystem of device innovation, active clinical research, and a reimbursement environment that shapes hospital and clinic purchasing decisions. In Europe, Middle East & Africa, heterogeneity across jurisdictions creates a complex mosaic of regulatory requirements and clinical standards; this regional diversity increases the importance of local clinical partnerships and adaptable regulatory strategies. In the Asia-Pacific region, rapid capacity expansion, strong medical device manufacturing infrastructure, and rising patient demand for both aesthetic and reconstructive procedures are driving intensified competition, accelerated product introductions, and a focus on cost-effective solutions.
Across these regions, differences in regulatory timelines, clinical training infrastructure, and distribution networks determine time-to-adoption and commercialization tactics. Consequently, manufacturers often deploy differentiated go-to-market strategies that prioritize regulatory harmonization, local clinical evidence generation, and supply chain resilience. Moreover, cross-regional learnings-such as clinical techniques validated in tertiary centers or material performance data collected in diverse patient populations-can be leveraged to refine product design and marketing messages, thereby accelerating acceptance across multiple geographies.
Competitive landscape analysis showing how portfolio breadth, targeted innovation, collaborations and strategic acquisitions are shaping differentiation and market positioning
Competitive dynamics within the nasal implant space are shaped by a combination of legacy device providers, specialized niche manufacturers, and emerging innovative entrants. Established firms typically emphasize a broad product portfolio, extensive clinical training programs, and strong distribution networks that reinforce hospital and clinic relationships. By contrast, smaller specialized companies often differentiate through focused design innovation, targeted clinical studies, and partnerships with key surgical opinion leaders to gain traction in specific procedure types or material segments.
Strategic collaborations and licensing arrangements between material scientists, device designers, and contract manufacturers are increasingly common, supporting faster time-to-market for novel polymer formulations or hybrid solutions that combine synthetic scaffolds with bioactive coatings. In parallel, consolidation through acquisitions remains a practical route for larger players to expand capabilities or enter new geographic markets, while venture-backed startups concentrate on disruptive approaches such as resorbable scaffolds or advanced surface treatments designed to reduce complications.
To succeed, companies must align clinical evidence generation with real-world performance tracking, prioritize rigorous quality systems, and maintain agile supply chains. Those that invest in clinician training, transparent safety data, and customer-centric commercial models will be better positioned to secure durable preference among surgeons and institutional purchasers.
Actionable strategic moves for device manufacturers and clinical leaders to strengthen evidence, supply resilience, channel focus and regulatory alignment for sustained adoption
Industry leaders should pursue a set of coordinated actions to strengthen commercial resilience, clinical credibility, and operational agility. First, prioritize robust clinical evidence programs that include prospective outcome measures and real-world registries to demonstrate long-term safety and functional benefits; this will support payer discussions and institutional adoption. Second, invest in material science pathways that validate multiple sourcing options-such as dual-sourcing key polymers or qualifying autologous tissue workflows-to mitigate supply chain disruptions and tariff exposure.
Third, tailor go-to-market strategies by end-user type, recognizing that ambulatory surgical centers, hospitals, and specialty clinics have distinct purchasing processes and clinical staffing models; direct clinical education and procedural training should be aligned with these channel-specific needs. Fourth, develop flexible commercial arrangements that combine direct sales engagement with distributor partnerships to optimize geographic reach while preserving clinical support capacity. Fifth, proactively engage with regulatory bodies to align post-market surveillance expectations and streamline approvals for iterative design improvements.
Finally, pursue operational initiatives that improve manufacturing adaptability and cost transparency, including modular production lines, validated alternative-material pathways, and clear total cost-of-ownership analyses for purchasers. By executing on these priorities, industry leaders can enhance competitive differentiation while better managing short-term risks and long-term clinical trust.
A rigorous, multi-source methodological framework combining clinician interviews, literature synthesis, supply chain analysis and comparative material evaluation to underpin findings
The research methodology underpinning this analysis integrates multi-source evidence gathering, expert consultation, and systematic synthesis of clinical and commercial inputs. Primary qualitative inputs were obtained through structured interviews with practicing surgeons, clinical procurement leaders, and regulatory affairs specialists to capture real-world decision criteria, procedural nuances, and regulatory pain points. These perspectives were complemented by a targeted review of peer-reviewed clinical literature, device registries, and performance summaries that emphasize safety, complication profiles, and patient-reported outcomes.
Operational insights were informed by supply chain assessments and interviews with manufacturing and distribution experts to understand sourcing risks, lead-time variability, and channel economics. The methodology also incorporated comparative analysis of material properties and handling characteristics to evaluate trade-offs among autologous tissue, expanded polytetrafluoroethylene, and silicone solutions, including nuances related to bone graft and cartilage techniques.
Throughout the process, triangulation was applied to validate findings across multiple sources and to surface convergent themes rather than relying on isolated data points. Ethical considerations and data privacy standards were upheld in all primary engagements, and results were contextualized for regional regulatory differences and clinical practice variability to ensure relevance for diverse stakeholders.
Concluding synthesis that highlights the imperative for evidence-driven product design, supply resilience and regionally tailored commercialization to secure long-term clinical adoption
In sum, the nasal implant field is evolving toward greater clinical differentiation, material optimization, and evidence-driven adoption. Clinical advancements and patient expectations are driving demand for implants that balance aesthetic and functional outcomes while minimizing complications and facilitating faster recoveries. Concurrently, regulatory scrutiny and procurement priorities are steering manufacturers toward transparent safety data, robust post-market surveillance, and demonstrable operational value.
Supply chain considerations and potential policy shifts-such as tariff scenarios-underscore the need for flexible sourcing strategies and validated alternative materials to maintain continuity of supply and cost competitiveness. Regional differences in regulatory regimes and clinical practice patterns necessitate tailored market entry and evidence-generation strategies to accelerate adoption. Ultimately, manufacturers and clinical leaders that align product design, clinical evidence, distribution strategy, and regulatory planning will be best positioned to capture clinician preference and sustain long-term adoption.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Nasal Implants Market
Companies Mentioned
The key companies profiled in this Nasal Implants market report include:- Arthrex, Inc.
- ArthroCare Corporation
- Collagen Matrix, Inc.
- GC Aesthetics PLC
- Global Consolidated Aesthetics Ltd.
- Guangzhou Wanhe Plastic Materials Co., Ltd.
- Implantech Associates, Inc.
- Integra LifeSciences Holdings Corporation
- KLS Martin Group
- Medartis AG
- Medtronic plc
- Olympus Corporation
- Porex Corporation
- RTI Surgical Holdings, Inc.
- Silimed Indústria de Implantes Ltda.
- Smith & Nephew plc
- Stryker Corporation
- Surgiform Technology Ltd.
- W. L. Gore & Associates, Inc.
- Zimmer Biomet Holdings, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
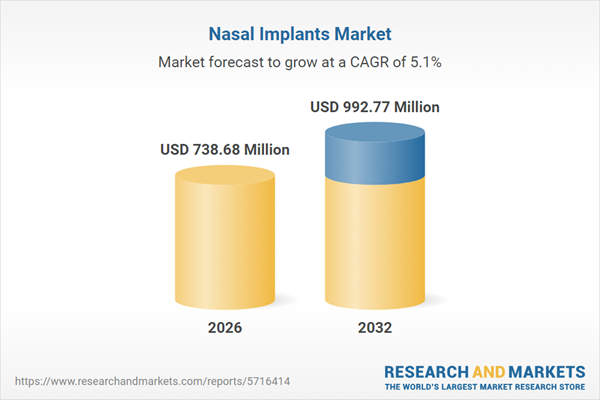
| Estimated Market Value ( USD | $ 738.68 Million |
| Forecasted Market Value ( USD | $ 992.77 Million |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |