Speak directly to the analyst to clarify any post sales queries you may have.
A comprehensive and strategically focused introduction to the technological, clinical, regulatory, and operational drivers shaping modern nuclear imaging equipment decisions
The nuclear imaging equipment landscape is at the intersection of rapid technological innovation, evolving clinical demand, and shifting regulatory priorities. Clinicians and procurement leaders alike face a complex set of choices as they evaluate planar scintigraphy imaging systems, positron emission tomography scanners, and single photon emission computed tomography scanners that increasingly offer hybrid capabilities. These modalities support a broad range of clinical pathways, from oncology staging to cardiac perfusion evaluation, and the equipment decision now extends beyond hardware to encompass radiopharmaceutical supply, workflow integration, and long-term service models.
As institutions balance capital allocation with clinical outcomes, the differentiation among systems depends not only on image quality but also on operational efficiency, dose optimization, and interoperability with digital infrastructure. Radiopharmaceuticals such as fluorodeoxyglucose, gallium-68, iodine-131, and technetium-99m play a central role in modality selection and clinical protocol design. Concurrently, the distribution of care across diagnostic imaging centers, hospitals and clinics, and research institutes shapes procurement timelines and service expectations. In this context, stakeholders must weigh clinical utility, staffing and training requirements, and regulatory compliance when selecting and deploying equipment.
Throughout this introduction, it is important to recognize that adoption decisions are multi-dimensional and that technology choices are increasingly influenced by clinical evidence generation, payer engagement, and the ability to integrate advanced analytics. By framing the conversation around clinical outcomes, operational resilience, and strategic partnerships, organizations can make informed decisions that support both immediate patient needs and longer-term innovation trajectories.
An in-depth analysis of the major technological, clinical, regulatory, and operational inflection points that are redefining nuclear imaging equipment deployment and value creation
The nuclear imaging ecosystem is experiencing transformative shifts that are reshaping how systems are developed, procured, and deployed. Advances in detector design and reconstruction algorithms have elevated the performance of positron emission tomography scanners and single photon emission computed tomography scanners, while hybrid integrations and software-driven enhancements are extending clinical applications. Concurrently, improvements in radiochemistry and the emergence of generator-produced isotopes are widening the clinical toolkit, yielding new diagnostic and therapeutic pathways that laboratories and clinics must operationalize.
Healthcare delivery models are evolving in parallel, with outpatient diagnostic imaging centers expanding capacity and research institutes accelerating translational studies that drive clinical adoption. Hospitals and clinics are balancing inpatient demands with opportunities to shift procedures to lower-cost settings, a trend that places a premium on compact, efficient systems that fit diverse facility footprints. At the same time, regulatory frameworks and reimbursement policies are adapting to novel imaging agents and combined diagnostic-therapeutic approaches, compelling manufacturers and providers to build stronger evidence packages and engage proactively with payers.
Digital transformation and artificial intelligence are further accelerating change by enabling automated workflows, enhanced image post-processing, and decision support that improves throughput and diagnostic consistency. Supply chain resilience has become a strategic priority, prompting reconsideration of sourcing strategies and service models to reduce downtime risk. Collectively, these forces are creating a market dynamic where clinical value, operational agility, and ecosystem partnerships determine long-term success, and where stakeholders who align technology, clinical pathways, and commercial strategies will capture the greatest benefit.
A pragmatic and evidence-based appraisal of how 2025 tariff measures have influenced supply chain strategy, procurement behavior, and clinical continuity across the nuclear imaging ecosystem
The introduction of tariff measures in the United States in 2025 has had a tangible influence on the economics and logistics of nuclear imaging equipment and radiopharmaceuticals. Increased cross-border costs and administrative complexity have prompted manufacturers, distributors, and providers to reassess procurement strategies and total cost of ownership for planar scintigraphy imaging systems, positron emission tomography scanners, and single photon emission computed tomography scanners. In response, vendors and healthcare organizations have accelerated efforts to localize supply chains, diversify manufacturing footprints, and negotiate long-term service agreements to mitigate price volatility and protect uptime.
Radiopharmaceutical supply chains felt particular pressure as tariffs affected inputs and transportation costs for isotopes and generators. Fluorodeoxyglucose production centers and gallium-68 generator suppliers faced higher landed costs, which influenced scheduling practices and pushed some institutions to strengthen in-house radiochemistry capabilities or form regional consortia for isotope production and distribution. Iodine-131 and technetium-99m logistics also required new contingency planning to secure uninterrupted access for diagnostic and therapeutic procedures. As a consequence, many clinical sites instituted stricter inventory controls, adjusted protocol sequencing, and prioritized cases based on clinical urgency and agent availability.
Moreover, the tariffs catalyzed a shift in commercial negotiations and contracting behavior. Procurement teams increasingly sought bundled solutions that included equipment, consumables, and service to stabilize long-term budgets. Manufacturers responded by offering modular upgrade paths, local service hubs, and financing instruments to maintain competitiveness. Regulatory compliance and customs processes added administrative overhead, encouraging collaboration between industry, institutional procurement, and government agencies to streamline imports for critical medical technologies. Overall, the cumulative impact reinforced the importance of supply chain resilience, flexible contracting, and operational adaptability across clinical and research settings.
A detailed segmentation-driven perspective linking product types, radiopharmaceutical usage, clinical applications, and end-user requirements to strategic procurement and deployment choices
A granular understanding of segmentation is essential for aligning product strategy and service models with clinical needs and institutional workflows. When framed by product, stakeholders must evaluate planar scintigraphy imaging systems for routine radionuclide studies, consider positron emission tomography scanners for high-sensitivity metabolic and molecular imaging, and weigh single photon emission computed tomography scanners for versatile functional imaging. The Single Photon Emission Computed Tomography category further differentiates into hybrid SPECT imaging systems that combine morphological and functional data for complex diagnostic pathways and standalone SPECT imaging systems that offer focused capability for established protocols.
Radiopharmaceutical considerations strongly influence modality selection and scheduling. Fluorodeoxyglucose remains a cornerstone tracer for oncologic and metabolic imaging, while gallium-68 has emerged as a key agent for targeted neuroendocrine and prostate imaging workflows. Iodine-131 continues to be central to therapeutic regimens as well as diagnostic follow-up in endocrine applications, and technetium-99m maintains broad utility across cardiology, infection imaging, and general nuclear medicine studies. These agents present distinct production, storage, and regulatory requirements that affect logistical planning and staff competencies.
Clinical application segmentation clarifies where investment yields the greatest patient and operational benefit. Cardiology programs rely on high-throughput perfusion imaging and robust quantification, infectious disease diagnosis requires sensitive whole-body protocols and rapid turnaround, neurology programs demand advanced kinetic modeling and motion correction, oncology initiatives benefit from combined metabolic and anatomic information, and orthopedic imaging leverages targeted bone imaging agents. Finally, end-user segmentation highlights how decision drivers differ between diagnostic imaging centers that emphasize throughput and cost efficiency, hospitals and clinics that balance acute care demands with specialized services, and research institutes that prioritize flexible, upgradeable platforms for protocol development and clinical trials.
A focused regional appraisal of how adoption patterns, supply chain strategies, reimbursement environments, and clinical priorities diverge across the Americas, EMEA, and Asia-Pacific
Regional dynamics shape technology adoption pathways, reimbursement frameworks, and supply chain choices, producing distinct opportunities and constraints across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, established clinical networks and advanced reimbursement mechanisms support widespread adoption of positron emission tomography and hybrid SPECT systems, while research institutions drive early clinical validation of novel radiopharmaceuticals. This region also emphasizes integrated service models and outcome-based evidence to support capital investment decisions.
The Europe, Middle East & Africa region presents a heterogeneous landscape where regulatory harmonization and cross-border collaboration impact access to advanced imaging agents and equipment. In several markets within this region, constrained capital budgets and divergent reimbursement rules encourage modular upgrades, extended service contracts, and public-private partnerships to expand access. Meanwhile, centers of excellence continue to invest in high-end PET installations and theranostic programs that require sophisticated radiochemistry and clinical trial infrastructure.
Asia-Pacific demonstrates rapid infrastructure investment, growing clinical expertise, and strong demand for both high-throughput systems in urban centers and compact solutions for distributed care settings. This region has been particularly active in localizing radiopharmaceutical production and investing in domestic manufacturing capabilities to reduce dependency on international supply chains. Across all regions, interoperability, workforce training, and policy engagement remain pivotal factors that determine the pace at which new technologies and agents are integrated into routine care.
A concise yet comprehensive assessment of competitive positioning, partnership models, product upgrade strategies, and service differentiation shaping industry leadership
Competitive dynamics in nuclear imaging equipment and radiopharmaceuticals reflect a balance between global platform providers, specialized device innovators, and regional manufacturers. Leading system suppliers compete on image performance, lifecycle service, and software ecosystems that enable quantitative imaging and AI-enabled workflows. Partnerships between device firms and radiopharmaceutical producers have increased, aligning tracer availability with scanner deployments and enhancing bundled value propositions for clinical networks.
Strategic activity has emphasized modular product roadmaps that allow clinical sites to upgrade detector technologies, integrate hybrid imaging capabilities, and adopt advanced reconstruction methods without full system replacement. Service differentiation has become a critical competitive lever, with extended warranties, remote diagnostics, predictive maintenance, and training programs reducing operational risk for clinical customers. At the same time, smaller innovators focus on niche opportunities such as compact PET designs, generator-based isotope solutions, and process innovations in radiochemistry to improve on-site production feasibility.
Mergers, alliances, and distribution agreements continue to reshape market access and aftermarket support footprints. Suppliers that can demonstrate robust clinical evidence, streamlined regulatory pathways, and local service capability are better positioned to secure long-term institutional relationships. Finally, companies investing in digital platforms that facilitate imaging analytics, clinical trial support, and multi-site data harmonization gain strategic advantage as healthcare systems prioritize outcomes, efficiency, and scalable innovation.
Practical strategic recommendations that industry leaders can implement to enhance supply chain resilience, clinical value realization, and commercial sustainability in nuclear imaging
Industry leaders can translate insight into action by pursuing a set of focused strategies that address clinical needs, operational resilience, and commercial viability. First, organizations should strengthen supply chain resilience by diversifying supplier networks, investing in regional production capacity for sensitive inputs, and establishing strategic inventory and distribution agreements to ensure continuity of isotopes and critical components. These steps reduce vulnerability to border-related cost pressures and logistical disruptions.
Second, companies should accelerate investments in software and analytics to improve workflow efficiency and diagnostic consistency. Integrating artificial intelligence for image reconstruction, lesion detection, and quantitative reporting enhances throughput and supports evidence generation for payers. In parallel, vendors and providers should co-develop outcome-focused clinical studies that demonstrate value across cardiology, oncology, neurology, infectious disease diagnosis, and orthopedic applications, thereby aligning technology features with reimbursement considerations.
Third, adopt flexible commercial models that bundle equipment, consumables, and performance-based service components to stabilize long-term costs for purchasers. Develop comprehensive training and credentialing programs to address workforce gaps and maximize the clinical utility of advanced systems. Finally, engage proactively with regulators and payers to streamline approval pathways and reimbursement policies for new radiopharmaceuticals and hybrid imaging protocols. By combining operational agility with clinical validation and collaborative commercialization, leaders can capture enduring strategic advantage.
A transparent, multi-method research approach combining expert interviews, secondary evidence triangulation, and scenario analysis to ensure robust and actionable findings
The research methodology underpinning this analysis combines qualitative and quantitative approaches to ensure rigor, relevance, and reproducibility. Primary research included structured interviews and consultations with a representative cross-section of stakeholders such as hospital procurement leads, nuclear medicine physicians, radiopharmacists, equipment service managers, and regulatory experts. These engagements provided firsthand perspective on procurement priorities, operational constraints, and clinical adoption drivers across different end-user categories.
Secondary research incorporated peer-reviewed literature, regulatory guidance documents, company technical disclosures, and public filings to validate product capabilities, clinical evidence, and technological roadmaps. Data synthesis used triangulation techniques to reconcile differing inputs and to surface consistent themes across sources. The methodology applied scenario analysis to examine supply chain stressors and policy impacts, while sensitivity checks ensured that qualitative inferences remained robust under a range of plausible operational conditions.
Quality assurance measures included expert peer review, cross-validation of technical claims with independent clinical sources, and iterative revisions to reflect emerging regulatory changes. The research approach prioritized transparency in assumptions and documented the provenance of major findings so that stakeholders can trace the basis for strategic recommendations and apply the insights to their specific organizational contexts.
A succinct and authoritative conclusion synthesizing technological advances, operational resilience, clinical validation, and strategic partnership imperatives for sustained value
In conclusion, nuclear imaging equipment and its supporting reagent ecosystem are undergoing substantive change driven by technology innovation, evolving clinical protocols, and shifting commercial dynamics. The confluence of improved detector performance, expanded radiopharmaceutical options, and digital analytics is enabling more precise diagnostic pathways and integrated therapeutic programs. As a result, healthcare providers must adopt a systems perspective that integrates device capability, tracer logistics, workforce training, and reimbursement strategy to maximize clinical impact.
Operational resilience and strategic partnerships have emerged as critical determinants of success. Supply chain reconfiguration, whether through regional production, strategic inventories, or service bundling, reduces vulnerability to policy and logistical disruptions. Meanwhile, vendors that invest in upgradeable platforms, robust service networks, and evidence-based value propositions will be best positioned to support diverse clinical settings ranging from high-throughput diagnostic imaging centers to specialized research institutes.
Looking forward, stakeholders who combine disciplined procurement, clinical validation, and proactive payer engagement will accelerate the adoption of advanced imaging modalities and tracers. By aligning technology investments with measurable clinical outcomes and operational priorities, providers and manufacturers can deliver greater value to patients while creating durable competitive advantage.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Nuclear Imaging Equipment Market
Companies Mentioned
The key companies profiled in this Nuclear Imaging Equipment market report include:- Absolute Imaging Inc.
- Advanced Accelerator Applications S.A. by Norvatis
- Agfa-Gevaert N.V
- Bayer AG
- Bozlu Holding A. Ş.
- Bracco Imaging S.p.A.
- Canon Medical Systems Corporation
- CMR Naviscan Corporation
- Cubresa Inc.
- DDD-Diagnostic A/S
- Digirad Corporation
- Edge Medical Solutions Private Limited
- GE HealthCare Technologies Inc.
- Koninklijke Philips N.V.
- Mediso Ltd.
- MR Solutions Ltd.
- Neusoft Medical Systems Co., Ltd.
- PerkinElmer Inc.
- Revvity Inc
- Rigaku Corporation
- Shimadzu Corporation
- Siemens AG
- Surgiceye GmbH
- United Imaging Healthcare Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
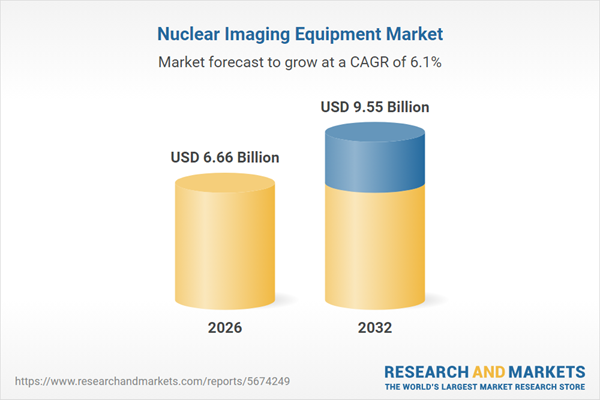
| Estimated Market Value ( USD | $ 6.66 Billion |
| Forecasted Market Value ( USD | $ 9.55 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |