Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introduction to nucleic acid sample preparation that illuminates how technological advances and operational priorities reshape laboratory performance and procurement
Introduction to Nucleic Acid Sample Preparation: Convergence of Technology, Clinical Demand, and Operational Resilience
Nucleic acid sample preparation sits at the nexus of molecular biology workflows, underpinning diagnostics, research, and biomanufacturing. Advances in extraction chemistry, automation platforms, and integrated workflows have elevated the reliability and throughput of downstream assays, enabling faster turnaround times and more reproducible results. At the same time, evolving clinical and regulatory requirements demand methods that are robust, contamination resistant, and traceable across diverse laboratory environments.Laboratories now balance competing priorities: maximizing sample integrity, scaling capacity for high-volume testing, and maintaining cost-effectiveness. These priorities drive investments across instruments, kits, reagents, and services, while also shaping preferences for manual versus automated processes. As a consequence, procurement strategies are becoming more strategic, with a greater emphasis on supplier resilience, validated workflows, and vendor ecosystems that support assay optimization and regulatory compliance.
This report frames the contemporary landscape by connecting technological capability with use-case demands. It highlights how incremental improvements in extraction efficiency translate into meaningful gains in downstream sensitivity and specificity, and how automation influences both operational metrics and personnel allocation. The introduction sets the stage for deeper discussion on transformative shifts, tariff impacts, segmentation nuances, regional dynamics, competitive positioning, and recommended actions for stakeholders seeking to navigate a fast-evolving environment.
How automation, reagent innovation, and expanding clinical applications are jointly transforming nucleic acid preparation workflows and supplier value propositions
Transformative Shifts Reshaping Nucleic Acid Sample Preparation Across Technology, Automation, and Clinical Application
The landscape of nucleic acid sample preparation has undergone decisive transformation driven by three interrelated vectors: automation, chemistry innovation, and the expansion of clinical and research applications. Automation has migrated from custom, high-cost platforms to modular, scalable systems that reduce manual variability and shorten hands-on time. This migration has heightened expectations for interoperable workflows where instruments, consumables, and software combine to deliver predictable performance across sites and users.Chemistry innovations, including refined binding resins and optimized lysis and wash buffer formulations, have improved yield and purity for challenging sample types such as low-titer viral specimens and degraded clinical material. These reagent-level improvements often unlock previously infeasible assays or extend the dynamic range of existing tests, thereby magnifying the impact of seemingly incremental product enhancements. Concurrently, extraction kits and protocols have evolved to address nucleic acid integrity concerns and to support multiplexed downstream assays, which increases the importance of validated, end-to-end workflows.
Clinical and research demand is also diversifying. Emerging applications in personalized medicine and genomic research require methods that can handle minute sample volumes while preserving long fragments for sequencing. Forensic and industrial laboratories prioritize robustness and contamination control, while high-throughput clinical diagnostics favor fully automated systems that integrate seamlessly with laboratory information systems. As a result, the industry is witnessing tighter integration between hardware vendors and reagent manufacturers, greater emphasis on standardized protocols, and growing interest in contract extraction services to manage variable throughput without capital overcommitment.
Taken together, these shifts reframe value propositions across the ecosystem. Suppliers that deliver validated, interoperable systems and flexible service models are gaining preference among end users who seek to de-risk adoption while accelerating time-to-result. Meanwhile, continued pressure to reduce per-sample costs and improve reproducibility is stimulating cross-portfolio innovation from manual consumables to fully automated extractors.
Comprehensive examination of how 2025 tariff adjustments altered sourcing strategies, supplier behavior, and operational continuity across nucleic acid preparation value chains
Cumulative Impact of United States Tariffs in 2025 on Supply Chains, Sourcing Decisions, and Commercial Strategies
Tariff changes in 2025 introduced layered complexity to procurement and supply-chain management for laboratories and suppliers of nucleic acid sample preparation products. Increased duties on certain imported laboratory instruments and consumables amplified landed costs and prompted buyers to reassess vendor relationships, inventory policies, and manufacturing footprints. In response, some organizations accelerated diversification of supply sources and increased nearshoring or dual-sourcing to mitigate tariff exposure and logistical uncertainty.Manufacturers and distributors adjusted by re-evaluating component sourcing and by revisiting price structures for instruments, kits, and reagents. For products with tightly integrated hardware-software ecosystems, vendors focused on clarifying total cost of ownership rather than simply offsetting tariffs through list-price increases. Strategic partnerships and contract manufacturing agreements emerged as practical routes to preserve access to critical consumables while optimizing cost bases.
Operationally, laboratories moderated ordering cadence and increased buffer inventories for high-volume consumables to smooth the impact of periodic price volatility. Contract research organizations and service providers responded by offering hedged pricing or fixed-term agreements to shield end users from short-term tariff-driven fluctuations. Regulatory and compliance considerations further complicated supply choices, as substitution of reagents or rapid changes in kit suppliers can necessitate revalidation of assays and documentation updates.
Overall, the tariff landscape in 2025 reinforced the need for greater supply chain transparency, proactive sourcing strategies, and collaborative commercial models. Firms that invested in flexible manufacturing, alternative sourcing corridors, and stronger supplier partnerships were better positioned to maintain continuity, preserve margins, and meet customer expectations amidst elevated trade friction.
In-depth segmentation synthesis revealing how product architecture, workflow choices, applications, end-user profiles, and automation preferences determine procurement and adoption dynamics
Key segmentation insights derived from product, workflow type, application, end user, and automation perspectives to guide tactical and strategic prioritization
A nuanced view of the market emerges when we consider five complementary segmentation lenses. The product perspective distinguishes instruments, kits, reagents, and services, where instruments encompass automated extractors, centrifuges, and vacuum manifolds that serve as backbone hardware for diverse workflows. Kits are differentiated into DNA extraction kits, RNA extraction kits, and viral nucleic acid extraction kits designed for specific assay needs. Reagents cover binding resins, elution buffers, lysis buffers, and wash buffers that together determine extraction efficiency and downstream compatibility. Services include contract research and custom extraction services that enable flexible capacity and method development.Examining workflow type clarifies that DNA extraction, microbial extraction, plasmid extraction, RNA extraction, and viral nucleic acid extraction each present distinct technical challenges and validation requirements. Within DNA and RNA workflows, column-based, magnetic bead-based, and organic extraction approaches offer trade-offs in throughput, purity, and automation suitability. Microbial extraction separates into chemical and mechanical lysis approaches, while plasmid extraction commonly relies on alkaline lysis or SDS-based methods. Viral nucleic acid extraction trends toward column-based and magnetic bead-based methods that maximize sensitivity and maintain sample integrity.
Application-driven segmentation highlights clinical diagnostics, drug discovery and development, forensic analysis, genomic research, and personalized medicine as primary use cases that differentially value throughput, regulatory compliance, and traceability. End users range from academic research laboratories and clinical laboratories to forensic laboratories, industrial laboratories, and pharmaceutical and biotechnology companies, each bringing unique procurement cycles and validation expectations that influence product adoption patterns.
Finally, automation segmentation-fully automated, manual, and semi-automated-frames how organizations trade off capital investment against throughput and labor efficiency. Fully automated systems appeal to high-throughput clinical and commercial labs focused on standardization and operator-independent performance, whereas manual and semi-automated options remain attractive to research and low-volume environments that prioritize method flexibility and lower upfront costs. Together, these segmentation lenses explain why suppliers that offer modularity across product types and robust validation support tend to secure cross-segment adoption and longer customer lifecycles.
Strategic regional analysis explaining how Americas, Europe Middle East & Africa, and Asia-Pacific differences influence demand patterns, supplier strategies, and adoption velocities
Regional dynamics and strategic implications across the Americas, Europe Middle East & Africa, and Asia-Pacific markets for nucleic acid sample preparation
Regional differences shape both demand patterns and strategic priorities. In the Americas, clinical diagnostics and biotechnology investments continue to drive demand for automated extractors and high-performance kits, with procurement decisions often influenced by reimbursement frameworks and centralized laboratory networks. The Americas region also emphasizes rapid adoption of innovative assays and close collaboration between instrument suppliers and large clinical laboratories to validate integrated workflows.Within Europe, Middle East & Africa, regulatory harmonization and diverse healthcare infrastructures produce heterogeneous adoption rates. Established research hubs and regional reference laboratories prioritize validated, interoperable systems that support multi-site studies, while emerging markets focus on cost-effective kits and reagent formulations that deliver acceptable performance within constrained budgets. Cross-border collaborations and pan-regional procurement initiatives often accelerate the uptake of standardized platforms.
Asia-Pacific exhibits strong growth momentum driven by large-scale public health testing programs, expanding academic research, and robust manufacturing ecosystems. Demand in this region favors scalable automation, locally manufactured consumables, and flexible service models to support variable throughput needs. Regional suppliers that combine global quality standards with localized support networks often gain traction, and strategic alliances between global vendors and regional manufacturers are common to balance cost, compliance, and supply chain resilience.
Taken together, these regional dynamics suggest that market entrants and incumbents must tailor go-to-market strategies to accommodate regulatory expectations, capital access, and laboratory consolidation trends while ensuring reliable local support and manufacturing agility to meet diverse regional needs.
Company-level perspectives on how innovation, partnerships, and service models create competitive differentiation and long-term customer retention in nucleic acid preparation
Competitive and company-level insights focused on innovation leadership, partnership models, and service diversification among incumbents and challengers
Company strategies in nucleic acid sample preparation diverge along axes of integrated system offerings, reagent specialization, and service provisioning. Incumbent instrument manufacturers often seek to expand consumable portfolios to lock in recurring revenue and to reduce customer friction through validated, end-to-end workflows. Conversely, reagent-focused firms invest in chemistry differentiation-improved resins and buffer systems-that can be licensed or bundled with third-party instruments to extend reach.Smaller challengers and specialized providers exploit niche opportunities by offering bespoke extraction kits or custom services tailored to demanding or emerging assay types. Contract research and extraction service providers further alter competitive dynamics by enabling rapid scaling for customers who prefer an asset-light model. Strategic partnerships, OEM agreements, and co-development programs are increasingly common mechanisms to accelerate market access, share validation burdens, and offer joint warranty or support contracts.
Another salient trend is the prioritization of software and data integration capabilities. Companies that provide laboratory information system interoperability, remote diagnostics, and predictive maintenance for automated extractors strengthen their value proposition by reducing total operational friction. In parallel, firms that emphasize regulatory documentation, validation packages, and training services differentiate themselves in heavily regulated clinical and forensic segments.
Overall, competitive advantage accrues to organizations that combine clear differentiation-whether through chemistry, automation, or services-with robust ecosystem partnerships and customer-centric support models that reduce adoption risk and enhance long-term retention.
Practical and prioritized recommendations for executives and product leaders to strengthen supply resilience, accelerate adoption, and differentiate through integrated services and automation
Actionable recommendations for industry leaders to capture value, reduce operational risk, and accelerate adoption of optimized nucleic acid preparation workflows
Leaders should prioritize integrated validation and interoperability when developing or selecting platforms, ensuring instruments, kits, and reagents are documented for consistent performance across representative sample types. This approach minimizes revalidation burden for customers and accelerates commercial adoption. Simultaneously, investment in modular automation that can scale from semi-automated to fully automated configurations will address diverse customer needs and protect against rapid obsolescence.Supply chain resilience must be elevated to a strategic imperative. Firms should assess alternate sourcing options, pursue regional manufacturing partnerships, and implement inventory strategies that reduce exposure to tariff-related and logistic disruptions. Transparent communication about component provenance and quality control practices will build trust with procurement and compliance teams, particularly in regulated healthcare settings.
Service differentiation provides a clear path to recurring revenue. Offering contract extraction services, method-development collaborations, and subscription models for consumables can smooth revenue cycles and increase customer touchpoints. Complementing products with digital solutions-LIMS integrations, remote support, and analytics that surface process deviations-further enhances value and reduces downtime for end users.
Finally, companies should align product roadmaps with emerging applications such as personalized medicine and high-sensitivity viral detection by investing in chemistry improvements that preserve nucleic acid integrity and in validation studies that demonstrate real-world performance. These combined steps will strengthen commercial positioning and provide defensible growth avenues in an increasingly competitive environment.
Transparent methodology explaining how multidisciplinary evidence, primary validation interviews, and triangulated analysis were combined to deliver robust market and technology insights
Research methodology detailing multidisciplinary data collection, validation processes, and the analytical framework used to derive insights and recommendations
This analysis synthesized qualitative and quantitative inputs derived from vendor documentation, peer-reviewed literature, regulatory guidance, and targeted interviews with laboratory directors, R&D leaders, and procurement specialists. Primary research included structured interviews to validate product positioning, performance attributes, and adoption drivers, while secondary research provided technical context for extraction chemistries, automation architectures, and regional regulatory considerations.Data triangulation ensured findings were corroborated across independent sources, with particular attention paid to assay performance parameters, interoperability claims, and service delivery models. Where proprietary claims were made by suppliers, validation steps included examination of published validation studies, regulatory filings, and user testimonials. Sensitivity analysis of strategic implications-such as tariff-driven sourcing shifts-relied on scenario-based reasoning and evidence from recent trade and procurement behaviors.
The analytical framework combined segmentation analysis with value-chain mapping to identify friction points and opportunity areas. This approach facilitated cross-sectional comparisons across product types, workflows, applications, end users, and automation levels, and supported the derivation of practical recommendations. Throughout, methodological transparency was maintained to enable readers to trace inference paths and to replicate specific elements of the evaluation for internal use.
Concise conclusion synthesizing strategic priorities for suppliers and end users to align innovation, validation, and supply resilience within nucleic acid preparation workflows
Concluding synthesis that crystallizes strategic imperatives for stakeholders navigating the evolving nucleic acid sample preparation ecosystem
Nucleic acid sample preparation remains a foundational element of contemporary molecular workflows, and its evolution will continue to influence the performance and economics of diagnostics, research, and biomanufacturing. Key imperatives for stakeholders include prioritizing interoperability, investing in reagent chemistry that addresses hard-to-extract sample types, and building resilient supply chains that can withstand trade and logistic volatility. Automation choices should be guided by throughput requirements and validation ecosystems rather than solely by upfront cost considerations.For suppliers, the path to sustained differentiation lies in offering validated, end-to-end solutions and in developing service models that lower customer adoption barriers. For end users, strategic procurement and careful validation protocols will reduce operational risk and improve assay reliability. Cross-sector collaboration-between instrument makers, reagent innovators, service providers, and end users-remains essential to accelerate technology diffusion and to ensure that emerging assays achieve clinical and research utility.
In short, organizations that align technical innovation with pragmatic supply and service strategies will be best positioned to capture value in a market characterized by rapid technological change and variable regulatory and trade headwinds.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Nucleic Acid Sample Preparation Market
Companies Mentioned
The key companies profiled in this Nucleic Acid Sample Preparation market report include:- Agilent Technologies, Inc.
- Aurora Biomed Inc.
- BGI Group
- Bio-Rad Laboratories, Inc.
- Bionano Genomics
- BIONEER CORPORATION
- Blue-Ray Biotech Corp.
- Danaher Corporation
- Eppendorf SE
- F. Hoffmann-La Roche Ltd
- Hamilton Company
- Illumina, Inc.
- LGC Biosearch Technologies
- Merck KGaA
- MP Biomedicals, LLC
- Nanjing Vazyme Biotech Co.,Ltd.
- Norgen Biotek Corp.
- Opentrons Labworks, Inc.
- PerkinElmer Inc.
- Promega Corporation
- QIAGEN N.V.
- RevoluGen Ltd
- Sansure Biotech Inc.
- Tecan Trading AG
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
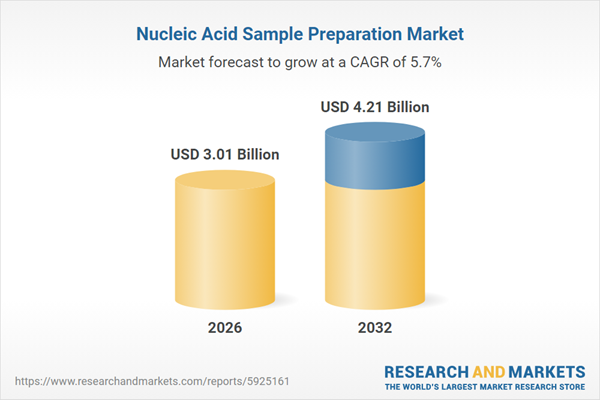
| Estimated Market Value ( USD | $ 3.01 Billion |
| Forecasted Market Value ( USD | $ 4.21 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |