Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite strong consumption levels, market growth faces a notable obstacle in the form of heightened scrutiny regarding the safety of long-term acid suppression therapy. Rising clinical concerns connecting prolonged use with adverse outcomes - such as bone fractures, micronutrient deficiencies, and kidney disease - have compelled healthcare practitioners to adopt more rigorous deprescribing strategies. These safety issues, coupled with fierce competition from low-cost generic substitutes, generate significant pressure on revenue generation for manufacturers operating in this sector.
Market Drivers
The primary engine of market growth is the rising global incidence of Gastroesophageal Reflux Disease (GERD) and peptic ulcers, a trend largely shaped by evolving lifestyles. As urbanization accelerates, populations are increasingly adopting sedentary habits and consuming processed foods, resulting in a spike in obesity-associated gastrointestinal conditions. This relationship reinforces the clinical demand for acid suppressants. The scale of this patient population is immense; the National Institutes of Health estimated the global pooled prevalence of GERD at roughly 13.98% in 2024. Moreover, metabolic health factors directly intensify this requirement; a study published on ResearchGate in January 2024, titled 'Obesity is associated with higher prevalence of gastroesophageal reflux disease,' found that obese patients had a 30% GERD prevalence compared to 24% in non-obese individuals, highlighting how weight-related issues necessitate therapeutic intervention.Simultaneously, the extensive market reach of affordable generic proton pump inhibitors has been fundamental in maintaining revenue streams. Since patent expirations, the availability of low-cost generic omeprazole has broadened access, establishing the drug as a staple in both prescription and over-the-counter markets. This affordability ensures that cost does not impede patient adherence, thereby keeping dispensation rates high. Usage volumes remain substantial; according to the 'Omeprazole - Drug Usage Statistics' database by ClinCalc in August 2025, omeprazole was the tenth most prescribed medication in the United States, with more than 45 million prescriptions written. This frequency confirms the drug's deep-rooted status as a standard of care, securing stable market performance despite downward pricing pressures.
Market Challenges
A pivotal challenge inhibiting the expansion of the Global Omeprazole Market is the intensified scrutiny surrounding the long-term safety of extended acid suppression therapy. As clinical data increasingly associates prolonged proton pump inhibitor use with serious adverse effects like bone fractures, micronutrient deficiencies, and chronic kidney disease, healthcare providers are actively adopting stricter deprescribing protocols. This modification in clinical practice emphasizes minimizing unnecessary medication usage, which directly restricts the volume of repeat prescriptions that have historically underpinned market revenue. The systematic move to re-evaluate long-term therapies necessitates a reduction in dispensing frequency, thereby curbing market growth.Recent data highlighting the widespread nature of unjustified prolonged usage reinforces this contraction. An evaluation by the Royal College of General Practitioners in 2025 revealed that approximately 62% of patients receiving continuous proton pump inhibitor treatment lacked a recorded medical indication for such extended therapy. This significant rate of inappropriate use compels medical boards and insurers to implement rigorous discontinuation policies. Consequently, manufacturers are encountering a tangible reduction in consumption rates as healthcare systems adjust prescription behaviors to align with safety guidelines, directly diminishing the sector's projected growth.
Market Trends
The Global Omeprazole Market is being significantly transformed by the development of patient-centric liquid formulations designed to meet the critical needs of pediatric and geriatric patients with dysphagia. Unlike conventional solid dosages, these innovative liquid solutions facilitate accurate dosing and enhance adherence for individuals unable to swallow capsules or tablets, establishing a specialized, high-value niche within the industry. This trend is defined by rising investment in production capabilities to support complex delivery systems that ensure drug stability. For example, in the 'ALKALOID AD Skopje 2025 BUSINESS PLAN - SUMMARY' from December 2024, Alkaloid AD Skopje forecasted a 10% increase in R&D expenditure to modernize its portfolio and aid the commercialization of patented value-added medicines, including its newly launched ready-to-use liquid omeprazole.At the same time, the creation of novel combination therapies is gaining traction as a method to address increasing antibiotic resistance in treating Helicobacter pylori infections. Manufacturers are engineering fixed-dose combinations that integrate omeprazole with advanced antibiotics to boost eradication rates and clinical effectiveness, advancing beyond basic acid suppression. This innovation enables companies to distinguish their offerings in a crowded market by providing comprehensive therapeutic regimens that streamline patient compliance. The commercial viability of these targeted treatments is demonstrated by financial performance; according to the 'RedHill Biopharma Announces First Half 2025 Financial Results' report from September 2025, net revenues for Talicia, an omeprazole-based combination therapy, rose to $3.8 million in the first half of 2025, highlighting the increasing market uptake of these specialized formulations.
Key Players Profiled in the Omeprazole Market
- Dr. Reddy's Laboratories Inc.
- Sandoz AG
- Perrigo Company plc
- Astrazeneca PLC
- Amneal Pharmaceuticals, Inc.
- Mylan N.V.
- Apotex, Inc.
- Watson Co., Ltd.
- Santarus Inc.
Report Scope
In this report, the Global Omeprazole Market has been segmented into the following categories:Omeprazole Market, by End-Use Application:
- Duodenal Ulcer
- Gastric Ulcer
- Gastroesophageal Reflux Disease
- Erosive Esophagitis
- Others
Omeprazole Market, by Sales Channel:
- Direct Sale
- Indirect Sale
Omeprazole Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Omeprazole Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Omeprazole market report include:- Dr. Reddy's Laboratories Inc
- Sandoz AG
- Perrigo Company PLC.
- Astrazeneca PLC
- Amneal Pharmaceuticals, Inc
- Mylan N.V.
- Apotex, Inc.
- Watson Co., Ltd..
- Santarus Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
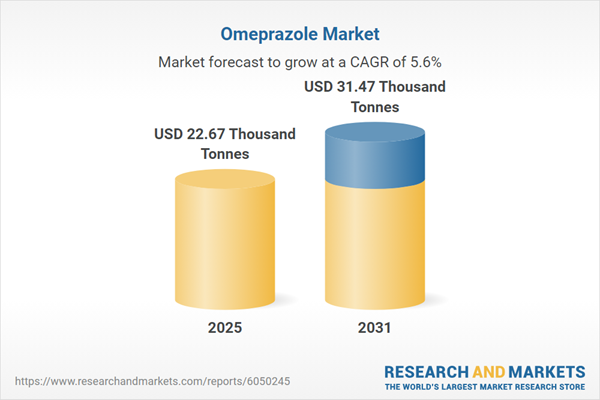
| Estimated Market Value ( USD | $ 22.67 Thousand Tonnes |
| Forecasted Market Value ( USD | $ 31.47 Thousand Tonnes |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |