Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Government-Funded Cell Therapy and Immunology Research
Governments worldwide are significantly increasing investments in cell and gene therapy, where PBMCs play a critical role. In the U.S., the National Institutes of Health (NIH) continues to back programs - such as immuno-oncology and vaccine research - that rely heavily on PBMC-based assays to characterize immune responses before and after experimental treatments.NIH-funded vaccine programs studying T-cell immunity routinely isolate PBMCs from donors to quantify immunogenicity in clinical testing. Similarly, in Europe, the Innovative Medicines Initiative (IMI) - a €2 billion public-private partnership co-funded by the European Commission - supports drug development platforms that use PBMC-based biomarker identification and immune monitoring in early-phase clinical trials. This large-scale public-sector support for PBMC-integrated therapeutics research underlines PBMCs’ indispensable role in translational medicine and offers a robust, policy-backed driver for market expansion.
Key Market Challenges
Regulatory and Quality Compliance Complexity
A significant challenge arises from the stringent and fragmented regulatory frameworks governing PBMC collection, processing, and distribution across jurisdictions. In the European Union, PBMC isolation facilities must conform to EU Tissue and Cells Directives (EUTCD) as well as national regulatory agency audits (e.g., Germany’s Paul-Ehrlich-Institut), with repeat compliance checks and data privacy enforcement under GDPR - violations can incur fines exceeding €20 million. In China, PBMC processing kits are treated as Class III medical devices requiring clinical validation, and export of PBMC samples with human genetic information is restricted under the 2021 Biosecurity Law, mandating dual approvals from NMPA and the Ministry of Commerce. These variable, complex regulations raise costs for cross-border research and discourage adoption in regions without harmonized oversight, inhibiting global standardization and scalable PBMC usage.Key Market Trends
Expanding Role in Vaccine Development and Immuno-Monitoring
A rapidly emerging trend is the expanded application of PBMCs for vaccine research and immunogenicity evaluation, especially in government-backed vaccine rollouts and pandemic preparedness. PBMCs offer critical insight into T-cell responses, cytokine profiles, and durability of immune memory. Governments funding large-scale vaccine trials - for diseases like COVID-19, malaria, tuberculosis - routinely incorporate PBMC-based assays into protocols to evaluate safety and immune efficacy. This creates predictable public-sector demand for standardized PBMC isolation, storage, and testing services, accelerating market growth tied to national immunization programs.Key Market Players
- Charles River Laboratories International, Inc.
- Lonza Group AG
- Corning Inc.
- Bio-Rad Laboratories Inc.
- ABCAM
- Biolegend Inc.
- ZEN-Bio Inc.
- DAPCEL, Inc.
- Creative Bioarray
- iXCells Biotechnologies USA, LLC
Report Scope:
In this report, the Global Peripheral Blood Mononuclear Cells Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Peripheral Blood Mononuclear Cells Market, By Product:
- Cryopreserved or Frozen PBMC
- Cultured or Fresh PBMC
- Peripheral Blood Mononuclear Cell Isolation & Viability Kits
Peripheral Blood Mononuclear Cells Market, By Application:
- Immunology
- Infectious Disease
- Hematology
- Others
Peripheral Blood Mononuclear Cells Market, By Technique:
- Density Gradient Centrifugation Process
- Leukapheresis
Peripheral Blood Mononuclear Cells Market, By Source:
- Human
- Animals
Peripheral Blood Mononuclear Cells Market, By Region:
- North America
- United States
- Mexico
- Canada
- Europe
- France
- Germany
- United Kingdom
- Italy
- Spain
- Asia-Pacific
- China
- India
- South Korea
- Japan
- Australia
- South America
- Brazil
- Argentina
- Colombia
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Peripheral Blood Mononuclear Cells Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Charles River Laboratories International, Inc.
- Lonza Group AG
- Corning Inc.
- Bio-Rad Laboratories Inc.
- ABCAM
- Biolegend Inc.
- ZEN-Bio Inc.
- DAPCEL, Inc.
- Creative Bioarray
- iXCells Biotechnologies USA, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | August 2025 |
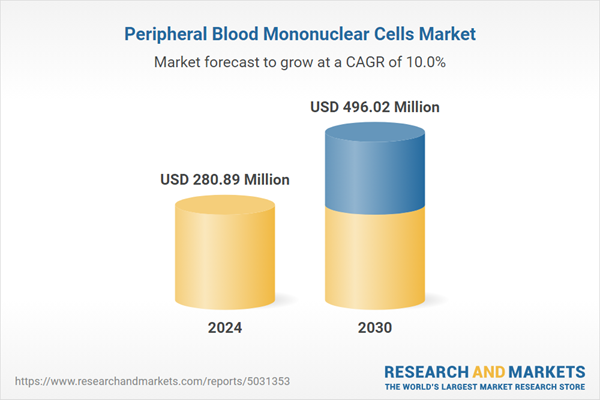
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 280.89 Million |
| Forecasted Market Value ( USD | $ 496.02 Million |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |