Speak directly to the analyst to clarify any post sales queries you may have.
The phytopathological disease diagnostics market is entering a new era as the need for accurate, timely, and scalable solutions becomes a non-negotiable imperative for food security and supply chain continuity. Stakeholders—including lab directors, agribusiness executives, and regulatory agencies—face a rapidly shifting diagnostic landscape marked by evolving technologies, new operational pressures, and changes in global trade.
Market Snapshot: Momentum in the Phytopathological Disease Diagnostics Market
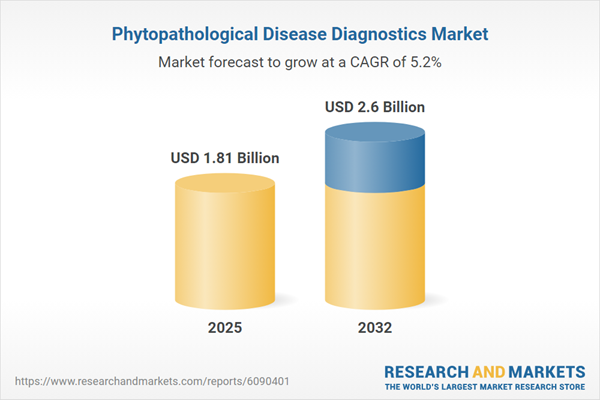
The phytopathological disease diagnostics market grew from USD 1.73 billion in 2024 to USD 1.81 billion in 2025 and is projected to maintain a CAGR of 5.24%, achieving USD 2.60 billion by 2032. This sustained growth mirrors ongoing investment in molecular diagnostics, robust commercial partnerships, and an emphasis on regional resilience. Recent advances have enabled wider pathogen coverage and earlier intervention, creating clear value for stakeholders determined to optimize crop health outcomes and procurement strategy.
Scope & Segmentation: Comprehensive Market and Technology Overview
This research delivers strategic segmentation and coverage across core dimensions, outlining product, methodology, and user requirements that define the competitive landscape:
- Diagnostic Method: Biochemical diagnostics, antibody detection, enzyme assays, immunoassays, cultural diagnostics, microbiological diagnostics, molecular diagnostics, serological diagnostics.
- Type of Disease: Bacterial, fungal, viral.
- Crop Type: Cereals and grains, fiber crops, fruits and vegetables, oilseeds and pulses, specialty crops.
- Distribution Channel: Offline retail, online retail.
- End-Users: Agricultural researchers, crop protection companies, farmers, government regulatory bodies, plant pathologists.
- Geographies: Americas (North America: United States, Canada, Mexico; Latin America: Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (Europe: United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland; Middle East: United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel; Africa: South Africa, Nigeria, Egypt, Kenya), and Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan).
- Key Companies Covered: Abbott Laboratories, Abingdon Health Ltd., Agdia, Inc., Agilent Technologies Inc., bioMérieux SA, BIOREBA AG, Creative Diagnostics, Inc., DiaSorin S.p.A., Envirologix Inc., Eurofins Scientific Inc., Leica Biosystems GmbH, LOEWE Biochemica GmbH, Luminex Corporation, Merck KGaA, Neogen Corporation, OptiGene Limited, PerkinElmer Inc., Promega Corporation, Qiagen N.V., Qualiplante SAS, Sartorius AG, Thermo Fisher Scientific Inc., TwistDx Limited.
Key Takeaways: Strategic Insights for Senior Decision-Makers
- Diagnostic workflows have transitioned toward rapid, molecularly informed approaches, improving response to disease threats for both centralized labs and field operations.
- Digital integration and laboratory automation are reducing the window between test results and actionable guidance, supporting more immediate agronomic interventions.
- Commercialization models are evolving, with diagnostic developers partnering across the value chain to deliver complete solutions—from assay design to decision-support analytics.
- Regulatory requirements for traceability and validation are prompting greater standardization and transparency across platforms and protocols.
- Segmentation by crop type, disease biology, and regional regulatory dynamics continues to drive tailored product features, distribution models, and service levels.
Tariff Impact: Navigating the Cost Structure and Supply Chain
Recent tariff adjustments have added complexity to global procurement for diagnostic reagents and equipment. Organizations that rely on imports have experienced increased lead times and costs, prompting a strategic focus on inventory management, local supplier development, and regional production. The market is responding by investing in modular, reagent-sparing workflows and diversifying sources to manage tariff-induced risk and maintain continuity.
Methodology & Data Sources
The research employs a mixed-methods approach combining systematic literature review, structured expert interviews, cross-checks via laboratory validation, and direct supply chain intelligence. Insights are grounded in both qualitative and quantitative datasets, featuring regulatory guidance and end-user perspectives for a holistic view.
Why This Report Matters: Actionable Value for Senior Leaders
- Guides investment decisions by illuminating emerging technology, localization strategies, and risk management priorities within the phytopathological disease diagnostics market.
- Enables targeted commercial strategy by revealing operational and regulatory differences across regions, disease classes, and user segments.
- Supports resilient procurement and supply planning by identifying tariff risks and scalable approaches to manufacturing and distribution.
Conclusion
Integrated diagnostic innovation, digital workflows, and supply chain resilience are reshaping plant health management. Senior decision-makers can leverage these insights to align technology adoption and partnership strategy for sustained crop protection and operational impact.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Phytopathological Disease Diagnostics market report include:- Abbott Laboratories
- Abingdon Health Ltd.
- Agdia, Inc.
- Agilent Technologies Inc.
- bioMérieux SA
- BIOREBA AG
- Creative Diagnostics, Inc.
- DiaSorin S.p.A.
- Envirologix Inc.
- Eurofins Scientific Inc.
- Leica Biosystems GmbH
- LOEWE Biochemica GmbH
- Luminex Corporation
- Merck KGaA
- Neogen Corporation
- OptiGene Limited
- PerkinElmer Inc.
- Promega Corporation
- Qiagen N.V.
- Qualiplante SAS
- Sartorius AG
- Thermo Fisher Scientific Inc.
- TwistDx Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.81 Billion |
| Forecasted Market Value ( USD | $ 2.6 Billion |
| Compound Annual Growth Rate | 5.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |