Speak directly to the analyst to clarify any post sales queries you may have.
Framing the clinical and technological context for platelet aggregation devices as diagnostics and research tools in an increasingly decentralized healthcare ecosystem
Platelet aggregation testing occupies a critical intersection of clinical diagnostics, translational research, and point-of-care decision-making. As therapeutic regimens that modulate hemostasis become more personalized and complex, the instruments and assays used to evaluate platelet function must deliver higher resolution, faster turnaround, and robust interoperability with clinical workflows. Advances in assay fidelity, miniaturized electronics, and software analytics are reshaping how clinicians and researchers interpret platelet behavior under pharmacologic and pathophysiologic conditions.Understanding this landscape requires attention to both established laboratory methodologies and emerging platforms that extend testing beyond central laboratories. The growth of cartridge-based systems and handheld devices reflects a push to democratize platelet function assessment while retaining analytical rigor. Meanwhile, innovations in flow cytometry and impedance approaches are enhancing specificity for platelet phenotypes and activation states. Together, these trends create new opportunities for clinical adoption, device differentiation, and collaborative research that spans academic, diagnostic, and industry stakeholders.
Identifying the convergent technological, clinical, and regulatory trends that are rapidly redefining product roadmaps and competitive dynamics in platelet testing
The platelet aggregation device landscape is undergoing transformative shifts driven by converging technological, regulatory, and clinical forces. On the technology axis, improvements in sensor design, microfluidic handling, and digital signal processing are enabling assays that previously required complex laboratory setups to be reimagined for point-of-care contexts. Concurrently, software-driven analytics and machine learning are enhancing assay interpretation, reducing operator dependence, and enabling longitudinal patient monitoring across care settings.Clinically, there is a clear move towards personalized antithrombotic management where platelet function testing informs dosing, perioperative planning, and therapy adjustments. This clinical demand is stimulating renewed investment in platforms that can deliver rapid, actionable results. Regulatory evolution is also influential; authorities are placing greater emphasis on real-world evidence, post-market surveillance, and clear demonstration of clinical utility. These combined shifts are encouraging strategic partnerships between device manufacturers, assay developers, and clinical networks to accelerate adoption and validate new use cases, thereby reshaping competitive dynamics and product roadmaps.
Examining how the 2025 tariff landscape is prompting supply-chain redesigns, regional manufacturing pivots, and strategic partnerships across the platelet device ecosystem
The imposition of new tariffs in 2025 has introduced a complex layer of cost and operational implications for stakeholders across the platelet aggregation device value chain. Manufacturers sourcing precision components, optical assemblies, and electronic modules from affected jurisdictions face immediate input-cost pressures that can cascade into higher list prices or compressed margins. In response, some suppliers are revisiting their supplier portfolios, accelerating qualification of alternative vendors, or negotiating longer-term contracts to stabilize input flows.Supply chain resilience has become a strategic priority. Companies with vertically integrated manufacturing or diversified supplier networks are better positioned to absorb tariff-related shocks, while smaller vendors and niche innovators may confront more pronounced margin erosion. The tariff environment is also incentivizing nearshoring and regional manufacturing shifts, as firms assess total landed cost, regulatory compliance complexity, and lead-time reliability. These adjustments influence product availability and procurement timelines for clinical laboratories and hospitals, prompting many buyers to increase lead-time buffers and explore multi-sourcing strategies.
Finally, tariffs are reshaping strategic partnerships. Licensing agreements, joint ventures, and localized manufacturing collaborations have emerged as mechanisms to mitigate tariff exposure while preserving market access. Regulatory approvals and quality systems continue to function as gatekeepers; however, pricing pressures introduced by tariffs are accelerating consolidation conversations and prompting firms to prioritize high-margin platforms and services while deferring lower-margin product investments.
Detailed segmentation perspectives that reveal how product types, test modalities, and end-user profiles drive distinct technical requirements and commercialization strategies
Actionable segmentation insights reveal where technological innovation and clinical demand intersect, and they inform prioritization for product development and go-to-market strategies. When viewed through the lens of product type, distinctions between benchtop instruments and portable instruments clarify trade-offs between analytical throughput, feature depth, and deployment environment. Benchtop units retain appeal for centralized labs that require higher throughput and expansive parameterization, whereas portable instruments are increasingly attractive for point-of-care deployment and decentralized clinical workflows.Analyzing test type shows distinct technical pathways and clinical applications. Flow cytometry, which encompasses direct and functional modalities, offers granular cellular and phenotypic resolution that supports research-grade assays and nuanced clinical interpretations. Impedance aggregometry, with platelet rich plasma impedance and whole blood impedance variants, provides robust, label-free measurements that integrate well with routine lab operations. Light transmission aggregometry, subdivided into optical density assays and turbidimetric assays, remains a reference methodology valued for its historical clinical validation and mechanistic clarity.
Modality-oriented segmentation highlights divergent commercialization routes. In vitro assays-including aggregometry systems and impedance systems-are often positioned for laboratory workflows and can be paired with extensive reagent ecosystems. Point-of-care systems-spanning cartridge-based solutions and handheld devices-prioritize usability, speed, and minimal sample preparation to support clinical decision-making in decentralized settings. Finally, end-user segmentation across academic institutions, diagnostic laboratories, hospitals, and research institutes illuminates distinct procurement drivers and validation requirements. Academic institutions, organized as teaching hospitals and universities, prioritize methodological flexibility and research partnerships. Diagnostic laboratories operating as central laboratories or point-of-care laboratories emphasize throughput, accreditation alignment, and cost-per-test. Hospitals, including community hospitals and tertiary centers, balance rapid clinical utility with operational integration. Research institutes, composed of biotechnology firms and pharmaceutical companies, seek high-resolution, reproducible assays that support drug development and translational research.
Cross-regional dynamics that influence regulatory pathways, commercialization timing, and manufacturing footprints across the Americas, EMEA, and Asia-Pacific
Regional dynamics are shaping where innovation is developed, validated, and adopted, and they influence regulatory timelines, reimbursement environments, and supply-chain configurations. In the Americas, there is a strong emphasis on clinical validation and integration with electronic health records, driven by health systems that prioritize measurable outcomes and real-world performance. This region also hosts substantial clinical research activity that accelerates adoption of advanced platelet function assays within both hospital and research settings.In Europe, Middle East & Africa, regulatory harmonization efforts and diverse reimbursement regimes require tailored market entry strategies. Countries with mature diagnostics ecosystems rapidly adopt platforms that demonstrate interoperability and established clinical utility, while emerging markets within the region present opportunities for cost-effective, portable solutions that address infrastructure variability. The Asia-Pacific region exemplifies a dual dynamic of rapid market growth and manufacturing capability. Several countries in this region are investing in domestic production and innovation ecosystems, which alters competitive positioning and can shorten time-to-market for locally developed devices. Cross-regional collaborations and technology transfer agreements are increasingly common as companies seek to balance regulatory complexity, tariff exposure, and proximity to clinical trial populations.
How incumbent manufacturers and agile innovators are differentiating through software analytics, consumable economics, and clinically validated use-case execution
Company strategies in the platelet aggregation device space reveal a mix of sustained core investments and targeted innovation plays. Incumbent instrument manufacturers continue to invest in incremental improvements-enhanced optics, refined fluidics, and expanded assay menus-while newer entrants focus on user experience, miniaturization, and integrated digital analytics. Strategic partnerships between device firms and diagnostic reagent providers are shaping bundled solutions that simplify adoption and create recurring revenue opportunities through consumables.Product differentiation increasingly depends on software capabilities: advanced signal processing, automated quality control, and connectivity to laboratory information systems. Firms that can demonstrate robust clinical evidence for specific use cases, such as perioperative monitoring or antiplatelet therapy management, gain traction with hospital clients and reference labs. At the same time, there is a clear niche for innovators designing compact, cartridge-based systems that reduce operator training and extend testing into emergency and outpatient settings. Competitive dynamics also reflect divergent regulatory strategies; some firms prioritize expedited pathway approvals for point-of-care solutions, while others invest in comprehensive validation to support broad laboratory adoption. Overall, company success hinges on the ability to align product performance with targeted clinical workflows and to offer credible post-market support and training that reduce barriers to clinician acceptance.
Practical strategic moves for device makers to strengthen product differentiation, supply-chain resilience, and clinical adoption across diverse care settings
Industry leaders seeking to sustain growth and resiliency should adopt a multi-faceted strategy that balances innovation with operational discipline. Prioritize modular platform designs that allow incremental feature upgrades without full instrument replacement; this approach reduces capital barriers for customers and extends product lifecycles. Complement hardware investment with software roadmaps that deliver automation, connectivity, and user-centric workflows that lower training burdens and enhance reproducibility. Strategic alliances with reagent and consumable partners can create integrated value propositions and predictable revenue streams from recurring purchases.On the supply-chain front, diversify sourcing and consider regional manufacturing partnerships to mitigate tariff-induced cost volatility and shorten lead times. Invest in rigorous clinical evidence generation that targets high-impact use cases-such as perioperative management and antithrombotic therapy monitoring-to accelerate payer acceptance and clinician uptake. Finally, embed post-market surveillance and training programs within commercial launches to ensure consistent performance across diverse clinical settings. These actions combined will strengthen market positioning, build trust with end users, and create defensible differentiation against both established competitors and emerging entrants.
A multi-layered research approach combining primary clinician input, expert validation, and systematic secondary synthesis to deliver reproducible, actionable findings
The research behind this analysis employed a layered methodology that integrates primary interviews, targeted expert consultations, and structured secondary intelligence to ensure rigor and relevance. Primary engagement included in-depth conversations with clinicians, laboratory directors, procurement officers, and device engineers to capture real-world operational constraints and adoption drivers. These qualitative insights were complemented by expert panels that validated key assumptions related to assay performance requirements, regulatory pathways, and clinical utility priorities.Secondary intelligence was synthesized from publicly available regulatory documents, clinical trial registries, patent filings, and company disclosures to map technological capabilities and innovation trajectories. Data triangulation techniques were used to reconcile differing perspectives and to highlight consensus viewpoints versus emerging hypotheses. Throughout the process, attention was given to methodological transparency and reproducibility: assumptions were documented, interview protocols standardized, and analytical frameworks stress-tested against alternative scenarios. This approach ensures that conclusions are grounded in diverse evidence sources and remain actionable for stakeholders navigating complex technical and regulatory environments.
Synthesis of strategic imperatives showing how technological, clinical, and geopolitical forces are jointly determining the future trajectory of platelet testing solutions
The platelet aggregation device ecosystem is at an inflection point where technological innovation, shifting clinical expectations, and geopolitical influences converge to reshape strategic priorities. Devices that successfully marry analytical rigor with operational simplicity are poised to gain adoption across centralized laboratories and decentralized clinical environments. Meanwhile, tariff dynamics and regional manufacturing shifts add urgency to supply-chain reconfiguration and partnership formation.Going forward, the most successful organizations will be those that invest selectively: prioritizing clinical evidence generation for high-impact use cases, building modular platforms that accommodate incremental upgrades, and expanding regional production footprints to manage cost volatility. By aligning product development with clear clinical workflows and embedding strong post-market support, stakeholders can reduce adoption friction and create sustainable value for providers and patients alike. The evolving landscape favors those who combine technical excellence with strategic flexibility.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Platelet Aggregation Devices Market
Companies Mentioned
The key companies profiled in this Platelet Aggregation Devices market report include:- Aggredyne, Inc.
- Bio/Data Corporation
- Chrono-Log Corporation
- Drucker Diagnostics, Inc.
- F. Hoffmann-La Roche Ltd.
- Haemonetics Corporation
- Hart Biologicals Ltd.
- Helena Biosciences Europe Ltd.
- Helena Laboratories Corporation
- Sentinel CH. SpA
- Siemens Healthineers AG
- Stago S.A.S.
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- Werfen, S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
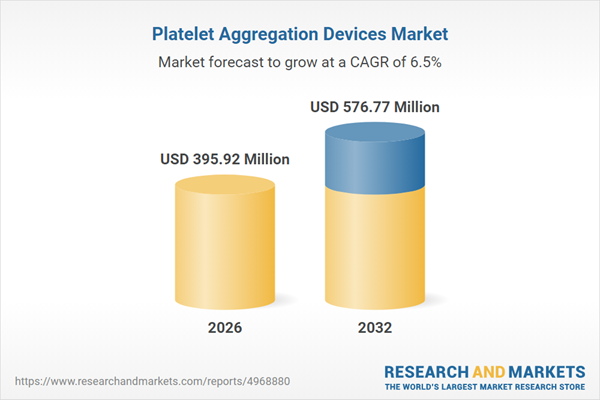
| Estimated Market Value ( USD | $ 395.92 Million |
| Forecasted Market Value ( USD | $ 576.77 Million |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |