Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive orientation to protein labeling fundamentals, experimental trade-offs, and operational considerations guiding laboratory and commercial decision-making
Protein labeling sits at the intersection of chemistry, biology, and instrumentation, powering analytical workflows that range from single-cell analysis to complex proteomic mapping. This introduction frames the technologies, user communities, and application demands that together govern adoption patterns and innovation pathways across academic, clinical, and industrial laboratories. Within this landscape, advances in labeling chemistries and detection platforms have repeatedly shifted experimental possibilities, enabling higher sensitivity, multiplexing, and compatibility with emerging platforms such as high-resolution mass spectrometry and advanced imaging.
Transitioning from foundational concepts to operational realities, laboratories now balance trade-offs between labeling specificity, preservation of native protein function, and downstream detection requirements. As experimental complexity grows, so does the need for standardized protocols and validated kits that reduce variability and accelerate time-to-result. At the same time, regulatory scrutiny around diagnostic uses and supply chain resilience for specialized reagents highlight the broader strategic considerations that decision-makers must evaluate when selecting labeling solutions. This introduction orients the reader to those tensions and sets the stage for deeper analysis of the transformative shifts, policy impacts, segmentation nuances, regional dynamics, and competitive tactics detailed in the subsequent sections.
How innovations in labeling chemistries, instrument sensitivity, and supply resilience are fundamentally reshaping protein labeling practices and procurement dynamics
Recent years have produced transformative shifts across the protein labeling landscape that are reshaping how laboratories prioritize reagents and workflows. Innovations in fluorescent chemistries and quantum dot formulations have driven higher multiplexing capabilities, while enzymatic conjugation techniques have improved labeling efficiency and reduced perturbation of native protein conformations. Together, these technological advances enable experiments that were previously impractical, supporting increasingly granular interrogation of cellular systems.
Concurrently, digital integration and instrument sensitivity improvements have heightened demand for labels that deliver consistent brightness, photostability, and minimal background. As a result, supplier strategies have shifted toward bundled solutions that include validated antibodies, optimized enzymatic kits, and detection-ready reagents to lower barriers to adoption. In parallel, regulatory and supply chain realities have incentivized development of labeling approaches that rely less on scarce raw materials, seeking alternatives that preserve performance while mitigating sourcing risk. Collectively, these changes are expanding the toolkit available to researchers, accelerating translational workflows, and prompting new collaborations between chemistry innovators, instrument manufacturers, and end users.
Assessment of how recent tariff-driven trade adjustments are altering reagent sourcing, production localization, and supply chain risk management for protein labeling stakeholders
Policy changes that alter import duties and trade terms create cascading effects across reagent availability, pricing structures, and supplier sourcing decisions, with tangible operational consequences for research and diagnostic programs. Tariff adjustments in a major market can increase landed costs for specialized components such as fluorescent dyes, quantum dot cores, and isotope precursors, prompting some suppliers to reassess manufacturing footprints or to localize critical production steps. This can reduce lead times for domestic customers but also concentrate upstream risk in new geographies.
Beyond direct cost implications, tariffs influence inventory policies and contractual terms. Laboratories and contract research organizations may extend inventory planning horizons or negotiate longer-term supply agreements to buffer against price volatility. In turn, suppliers may implement dual-sourcing strategies and increase transparency around origin-of-materials to reassure customers and preserve procurement continuity. Importantly, tariff-driven shifts also accelerate strategic investments in alternative technologies that rely less on tariff-exposed inputs, such as enzymatic labeling routes that reduce dependence on imported synthetic dyes or localized isotope labelling services that limit cross-border movement of regulated materials.
Taken together, these dynamics underscore the need for procurement and R&D leaders to reassess supplier risk profiles, evaluate the total cost of ownership for labeling workflows, and incorporate trade policy scenarios into sourcing and development roadmaps. While tariffs do not change the scientific fundamentals, they do alter the economics and operational practices that determine which labeling solutions win in real-world laboratory settings.
Integrated segmentation analysis revealing label chemistries, application workflows, product formats, end-user profiles, and technique-specific adoption drivers across the ecosystem
A nuanced segmentation perspective reveals where value and vulnerability coexist across the protein labeling ecosystem, informing where manufacturers and end users should focus development and commercialization efforts. Based on label type, the field encompasses affinity and biotin labeling approaches, enzymatic labeling-most notably alkaline phosphatase and horseradish peroxidase variants-fluorescent labeling that includes both organic dyes such as Alexa Fluor, FITC, and Rhodamine as well as quantum dot technologies represented by CdSe and InP cores, and isotopic labeling split between radioisotopes like 14C and 35S and stable isotopes such as 13C and 15N. Each label class brings specific handling requirements, detection compatibilities, and regulatory considerations that drive product design and customer support needs.
Based on application, workflows span flow cytometry with multi-color and single-color analyses, immunoassays including ELISA and Western blotting with subtypes such as competitive and sandwich ELISA and chemiluminescent versus colorimetric Western blot detection, mass spectrometry approaches exemplified by ESI-MS and MALDI-TOF, microscopy modalities like confocal and fluorescence microscopy, and proteomics disciplines divided into qualitative methods such as gel electrophoresis and mass spectrometry analysis and quantitative strategies like iTRAQ, SILAC, and TMT. Product offerings bifurcate into kits and reagents, with kits addressing fluorescent or radioactive labeling workflows and reagents covering enzymes, isotopic reagents, and labeling dyes. End users range from academic research institutes to biotechnology companies, contract research organizations, diagnostic laboratories, and pharmaceutical companies, each exhibiting distinct validation demands and procurement protocols.
Technique selection further differentiates market needs: direct labeling routes, whether chemical or enzymatic, prioritize throughput and minimal sample handling, while indirect approaches such as biotin-avidin interactions and secondary antibody labeling emphasize amplification and flexibility. Integrating these segmentation layers clarifies where technical innovation, regulatory support, and supply chain investments will deliver the greatest return and where targeted education and protocol standardization can unlock broader adoption.
How geographic differences in manufacturing capacity, regulatory complexity, and research infrastructure are shaping protein labeling availability and adoption patterns globally
Regional dynamics shape both access to labeling technologies and the strategic choices of suppliers and purchasers. In the Americas, innovation hubs and established reagent manufacturers support a dense network of academic and commercial end users with high demand for validated kits and high-throughput compatible reagents, while clinical and diagnostic adoption trends push suppliers to emphasize regulatory documentation and lot-to-lot consistency. Europe, Middle East & Africa features heterogeneous regulatory environments and a mix of well-established research institutions and emerging biotech clusters, prompting suppliers to offer versatile product lines and robust technical support to navigate diverse compliance frameworks.
Asia-Pacific combines rapid capacity expansion with an increasing emphasis on domestic manufacturing and localized research agendas, encouraging suppliers to optimize cost structures and invest in regional partnerships. Cross-region collaboration continues to drive technology transfer and harmonization of best practices, yet logistical considerations and local regulatory variations mean that suppliers typically deploy differentiated commercial strategies by geography. Consequently, organizations making procurement and development decisions must account for these regional nuances, balancing global consistency with the need for localized compliance, customer support, and supply chain resilience.
Competitive landscape dynamics showing how incumbent suppliers, niche innovators, and service-led strategies are redefining value propositions for labeling products and services
Competitive dynamics in the protein labeling arena reflect a blend of mature reagent suppliers, specialized niche innovators, and agile start-ups focused on discrete technologies such as quantum dots, isotopic labeling, or enzymatic conjugation. Established suppliers prioritize expanding validated workflows and offering bundled solutions that reduce time-to-result for end users, investing in quality systems and documentation to support diagnostic and clinical applications. Niche innovators frequently focus on high-performance chemistries or proprietary manufacturing processes that deliver superior brightness, photostability, or isotopic purity, enabling differentiation through technical advantages.
Across the competitive landscape, strategic behaviors include deepening collaborations with instrument manufacturers to ensure compatibility and co-marketing, pursuing licensing or exclusive supply relationships for key chemistries, and expanding service offerings to include custom labeling and protocol optimization. Many players also invest in application training, digital content, and localized technical service to lower adoption friction. For buyers, these company-level tactics translate into clearer value propositions: suppliers who combine technical excellence, regulatory readiness, and dependable logistics are positioned to capture long-term partnerships with high-value end users such as pharmaceutical developers and diagnostic laboratories.
Actionable strategic framework for suppliers and purchasers to enhance product modularity, regional resilience, technical service offerings, and regulatory readiness
Industry leaders should adopt a pragmatic, multi-pronged approach to capitalize on evolving scientific needs while mitigating operational risks. First, prioritize modular product architectures that allow customers to combine high-performance labels with validated kits and flexible reagent formats, thereby addressing diverse workflows without fragmenting internal manufacturing processes. Second, invest in regional manufacturing or qualified local partners to shorten lead times for critical inputs and to decrease exposure to trade policy volatility, while simultaneously maintaining centralized quality oversight to ensure consistency across markets.
Third, expand value through technical services such as custom conjugation, assay validation support, and protocol standardization that help end users reduce time-to-result. Fourth, cultivate instrument and consumable compatibility through co-development agreements, ensuring that labels are optimized for prevailing detection platforms. Fifth, reinforce regulatory capabilities by building robust documentation and third-party validations that accelerate adoption in clinical and diagnostic contexts. Finally, embed scenario-based supply chain planning into commercial strategy, using stress-testing exercises to anticipate raw material disruptions and to design contingency sourcing that preserves customer commitments.
Robust mixed-methods research approach combining expert interviews, technical literature synthesis, and supply chain scenario mapping to underpin actionable insights
This research synthesizes primary engagements with laboratory decision-makers and technical experts, targeted reviews of peer-reviewed literature and validated technical application notes, and a systematic evaluation of publicly available regulatory guidance and supply chain disclosures. Primary conversations focused on protocol adoption barriers, sourcing preferences, and validation requirements across academic, clinical, and industrial end users, enabling the identification of recurring pain points such as reproducibility challenges and reagent lead times. Secondary research included cross-referencing technical performance claims with independent method comparisons and manufacturer specifications to assess real-world compatibility and handling constraints.
Analytical methods prioritized qualitative synthesis to map technology adoption drivers, combined with thematic coding of stakeholder interviews to surface common strategic imperatives. Supply chain analysis incorporated origin-of-materials assessments and scenario mapping to illustrate where policy or logistical disruptions would exert the greatest operational impact. Throughout, the methodology emphasized triangulation across sources to ensure conclusions reflect convergent evidence rather than isolated anecdotes, and transparency around assumptions to support evidence-based decision making by commercial and technical stakeholders.
Synthesis of scientific advancements and operational realities highlighting the priorities necessary to sustain innovation, adoption, and supply reliability in protein labeling
Protein labeling continues to be a foundational enabler for modern biological research and diagnostic workflows, but its future trajectory will be determined as much by practical constraints-such as reagent availability, regulatory expectations, and integration with detection platforms-as by pure technological possibility. Advances in fluorescent chemistries, enzymatic conjugation, and isotope labeling expand analytical capabilities, while evolving procurement practices and regional manufacturing shifts alter how quickly those innovations translate into routine laboratory use. Stakeholders who align product design with operational realities, invest in robust validation and documentation, and anticipate supply chain shifts will secure durable advantages.
Looking ahead, coordination across chemistry innovators, instrument manufacturers, and end users will be essential to standardize protocols, reduce variability, and accelerate adoption of high-value labeling solutions. By marrying technical excellence with pragmatic supply and regulatory strategies, the community can simultaneously advance scientific capability and ensure that high-performance labeling tools remain accessible and reliable for the diverse set of applications that drive discovery and diagnostics.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Protein Labeling Market
Companies Mentioned
The key companies profiled in this Protein Labeling market report include:- Abcam plc
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- Danaher Corporation
- LI-COR Biosciences
- Merck KGaA
- PerkinElmer, Inc.
- Promega Corporation
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
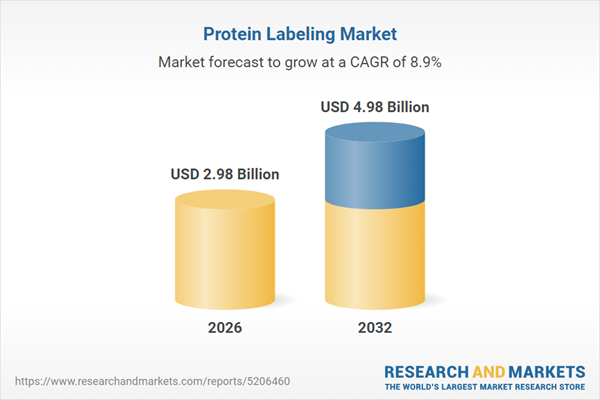
| Estimated Market Value ( USD | $ 2.98 Billion |
| Forecasted Market Value ( USD | $ 4.98 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |