Speak directly to the analyst to clarify any post sales queries you may have.
The quinidine sulfate market remains strategically important as clinical, regulatory, and supply chain considerations converge to influence access and patient outcomes. Senior decision-makers must navigate evolving therapeutic preferences, tightened regulatory scrutiny, and ongoing procurement complexities while ensuring safe and reliable delivery of this essential antiarrhythmic and antimalarial agent.
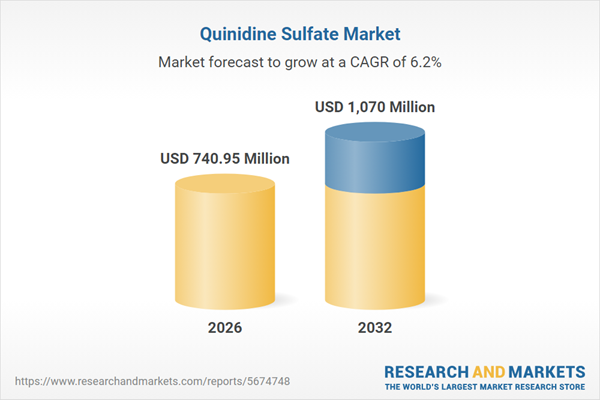
Market Snapshot: Quinidine Sulfate Market Growth and Dynamics
The Quinidine Sulfate Market grew from USD 708.99 million in 2025 to USD 740.95 million in 2026 and is projected to reach USD 1.07 billion by 2032 with a CAGR of 6.17%. Market expansion is driven by continued niche clinical roles, diversified sourcing strategies, and regional regulatory shifts. Competitiveness is shaped by both legacy suppliers and new entrants responding to escalated vigilance around safety, supply chain robustness, and optimized manufacturing practices.
Scope & Segmentation of the Quinidine Sulfate Market
- Application Areas: Antiarrhythmic treatment for severe cardiac arrhythmias and antimalarial therapy in specific clinical contexts.
- Dosage Forms: Availability in capsules, injectable solutions, and tablets, each demanding tailored manufacturing protocols and implementing form-specific stability or aseptic requirements.
- Distribution Channels: Hospital pharmacy, online pharmacy, and retail pharmacy settings, with contrasting inventory management approaches and regulatory compliance needs.
- End Users: Hospitals, clinics, and home healthcare environments, each shaping administration oversight, patient education strategies, and monitoring protocols.
- Regions Covered: Americas, Europe, Middle East & Africa, and Asia-Pacific, each with unique regulatory, operational, and procurement dynamics.
- Key Technologies: Modern manufacturing processes, robust quality control frameworks, advanced supply chain mapping, and pharmacovigilance systems for enhanced drug safety and production resilience.
Key Takeaways for Senior Stakeholders
- Quinidine sulfate’s distinctive therapeutic profile sustains its use where alternative agents are unsuitable, requiring specialized governance and vigilant clinical protocols.
- Safety concerns, such as QT interval prolongation and significant drug-drug interactions, necessitate centralized prescribing and enhanced monitoring by multidisciplinary teams.
- Manufacturers increasingly diversify active pharmaceutical ingredient sourcing and reinforce quality systems to mitigate risks of supply disruption and regulatory noncompliance.
- Procurement and clinical leaders must recalibrate strategies to address evolving regulatory requirements, risk communications, and periodic inspection schedules.
- Organizations that foster collaboration between commercial, clinical, and supply chain disciplines are better positioned to absorb regulatory changes and operational challenges.
Tariff Impact: U.S. Trade Policy and Supply Economics
United States tariff measures through 2025 have added layers of cost and complexity to quinidine sulfate procurement. Manufacturers responded by reassessing supplier location strategies, accelerating nearshoring where feasible, and diversifying vendor portfolios to buffer tariff volatility. These shifts prompted distributors and healthcare providers to renegotiate contracts and enhance supply chain visibility, ensuring continued patient access despite operational frictions arising from regulatory schedules and production relocations.
Methodology & Data Sources
This report applies a mixed-methods research approach including thorough secondary regulatory documentation review, pharmacovigilance analysis, and in-depth interviews with clinicians, pharmacists, procurement directors, and supply chain managers. Data integrity was ensured through cross-validation across multiple independent sources, while transparency was maintained via documented interview sampling and source hierarchies to support actionable operational insights.
Why This Report Matters for Decision-Makers
- Delivers fact-based analysis to support clinical, procurement, and regulatory strategies in the context of evolving market and policy environments.
- Guides leaders in aligning stewardship programs, diversifying supply chain approaches, and mitigating operational or safety risks linked to quinidine sulfate.
- Enables organizations to strengthen patient access and compliance by integrating regulatory intelligence, procurement best-practices, and contemporary pharmacovigilance frameworks.
Conclusion
Quinidine sulfate’s continued clinical relevance, paired with carefully managed supply partnerships and regulatory insight, ensures ongoing patient benefit and market stability. Success in this market depends on coordinated governance, supplier diversification, and vigilant clinical oversight.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Quinidine Sulfate Market
Companies Mentioned
The key companies profiled in this Quinidine Sulfate market report include:- Actiza Pharmaceutical Private Limited
- Akhil Healthcare Private Limited
- Alpspure Lifesciences Private Limited
- Atra Pharmaceuticals Private Limited
- Bennet Pharmaceuticals Ltd.
- Buchler GmbH
- Centurion Laboratories
- Cipla Limited
- Devlife Corporation Private Limited
- Dwarkesh Pharmaceuticals Private Limited
- Inga Laboratories Pvt. Ltd.
- Ipca Laboratories Ltd.
- Lark Laboratories Ltd.
- Merck & Co., Inc.
- Novartis AG
- PMC Group, Inc.
- Prism Industries Private Limited
- Teva Pharmaceutical Industries Ltd.
- USV Private Limited
- Vivan Life Sciences Private Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 740.95 Million |
| Forecasted Market Value ( USD | $ 1070 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |