Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative overview of the respiratory disease testing environment shaped by clinical pressures, innovation in diagnostics, and evolving care delivery models
The testing landscape for respiratory diseases stands at an inflection point where clinical necessity, technological progress, and systemic pressures converge. Rising prevalence of chronic respiratory conditions, episodic surges in infectious respiratory illnesses, and the persistent imperative to improve diagnostic accuracy have collectively heightened demand for sophisticated testing across clinical and community settings. Concurrently, innovation in molecular diagnostics, digital imaging, and immunoassays is enabling earlier detection and more precise disease characterization, which in turn shapes care pathways and resource allocation.As stakeholders adapt, the interplay between laboratory capabilities, point-of-care modalities, and at-home solutions is redefining how providers and patients approach diagnostic workflows. Moreover, an aging population and shifting epidemiology increasingly prioritize long-term monitoring and prognostic assessment alongside acute diagnosis. Therefore, leaders must reconcile the clinical potential of new technologies with pragmatic considerations such as interoperability, workforce training, and regulatory compliance. Taken together, these dynamics underscore why respiratory disease testing merits renewed strategic focus and why integrated, evidence-driven planning will determine which organizations capture clinical relevance and commercial opportunity
How converging advances in molecular diagnostics, digital imaging, and remote care are redefining respiratory testing pathways and value capture across the healthcare continuum
Over the past several years, transformational shifts have reconfigured the respiratory testing ecosystem from one driven primarily by episodic clinical demand to a landscape shaped by continuous data flows and technology-enabled care. Rapid advances in molecular diagnostics have improved sensitivity and turnaround times, while next-generation imaging and artificial intelligence have enhanced interpretive consistency and triage capabilities. Simultaneously, digital health platforms and telemedicine have broadened access to testing and enabled remote monitoring, meaning that diagnostic insight now informs care across a patient’s entire journey rather than at a single point in time.These changes have been reinforced by evolving regulatory frameworks that emphasize diagnostic validation, post-market surveillance, and data security, prompting manufacturers and laboratories to invest in compliance and quality systems. In addition, shifting procurement practices have prioritized integrated solutions that combine instruments, reagents, and software, fostering strategic partnerships across the value chain. Consequently, organizations that align clinical utility with streamlined implementation, scalable data integration, and robust quality governance are best positioned to translate technological promise into improved patient outcomes and sustained commercial performance
Assessment of how 2025 tariff revisions have reshaped procurement, supply continuity, and strategic sourcing within the respiratory testing supply chain
Policy adjustments to tariff regimes implemented in 2025 have introduced a new variable into the respiratory testing value chain, affecting procurement strategies, supplier relationships, and operational planning. Imports of diagnostic instruments, imaging equipment, and specialized reagents are particularly sensitive to changes in tariff structures, and laboratories and hospitals that rely on cross-border supply chains have had to reassess sourcing, inventory buffering, and vendor terms to preserve continuity of care. As a result, procurement teams increasingly evaluate total landed cost and lead time risk rather than unit price alone, and they seek contractual flexibility to mitigate future policy volatility.Moreover, tariff-related cost pressures can cascade into testing pathway decisions, influencing whether organizations prioritize in-house platforms, consolidate suppliers, or adopt reagent rental and service-based models. In parallel, research laboratories and manufacturers face higher input costs for imported components, prompting intensified efforts to localize production, qualify alternative suppliers, or redesign kits to use more readily available materials. From the patient perspective, providers may reconfigure testing algorithms to preserve access to essential diagnostics while managing affordability, which underscores the need for collaborative engagement among industry, payers, and regulators to ensure that trade policy objectives do not inadvertently constrain clinical care
Comprehensive segmentation-driven insights that delineate testing modalities, components, patient cohorts, technologies, clinical intents, disease categories, and end-user priorities
A granular view of market segmentation reveals distinct opportunities and operational imperatives across technical modalities, product components, patient demographics, and care settings. Based on test type, Blood Tests remain fundamental for biomarker assessment while Imaging Tests, which include Chest X-Ray and Computed Tomography, continue to play a central role in anatomical evaluation and staging, and Pulmonary Function Tests provide objective measures of respiratory performance that guide chronic disease management. Based on component, Instruments require capital planning and maintenance infrastructure, Reagents & Kits demand dependable supply chains and lot-to-lot consistency, and Software must prioritize interoperability, analytics, and regulatory compliance to realize clinical value.Based on age group, Adults constitute the majority of routine diagnostic demand while Geriatrics drive increasing needs for monitoring and comorbidity management, and Pediatrics often require tailored assays and noninvasive approaches. Based on technology, Imaging Technologies and Immunoassays serve complementary clinical use cases alongside Microbiology and Molecular Diagnostics, which deliver high specificity for infectious etiologies and genomic insights. Based on test purpose, Diagnosis, Monitoring, Prognosis, and Screening each impose different performance and workflow requirements, influencing the selection of platforms and reimbursement pathways. Based on disease type, common profiles include Asthma, Chronic Obstructive Pulmonary Disease with Chronic Bronchitis and Emphysema subtypes, Infectious Respiratory Diseases such as Influenza, Pneumonia, and Tuberculosis, and Lung Cancer, each requiring unique diagnostic algorithms. Based on end users, Diagnostic Laboratories emphasize throughput and accreditation, Home Care Settings prioritize portability and ease of use, Hospitals & Clinics require integrated workflow solutions, and Research Laboratories focus on flexibility and depth of analytical capability
Regional analysis highlighting how distinct regulatory regimes, infrastructure maturity, and procurement practices shape adoption and deployment across global markets
Regional dynamics exert a powerful influence on technology adoption, regulatory pathways, and reimbursement mechanisms, producing differentiated strategic imperatives across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, robust hospital networks and private laboratory infrastructure create demand for high-throughput platforms and advanced imaging solutions, while regulatory processes emphasize clinical evidence and quality systems that support adoption in acute care settings. Conversely, Europe, Middle East & Africa feature diverse regulatory environments and variable access to centralized laboratory services, which encourages modular solutions, local partnerships, and flexible financing models that can accommodate differing healthcare delivery realities.Asia-Pacific displays rapid adoption of molecular diagnostics and point-of-care technologies, driven by significant investments in public health infrastructure, large patient populations, and an appetite for digital integration. Across regions, trade policy, local manufacturing capacity, and clinical practice patterns shape procurement decisions and product configurations, leading companies to tailor commercial models and support services to regional nuances. Therefore, strategic planners must reconcile global product strategies with localized execution to achieve scale while respecting regulatory and operational diversity
Key competitive patterns showing how technological specialization, strategic partnerships, and evidence generation determine success across diagnostic instruments, reagents, and software
Competitive dynamics in respiratory disease testing are characterized by a blend of technological specialization, strategic partnerships, and an increasing emphasis on software-enabled differentiation. Firms that focus on diagnostic instruments typically invest in platform reliability, service networks, and consumable ecosystems to drive recurring revenue. Suppliers of reagents and kits prioritize supply resilience, assay performance, and ease of integration, while software developers concentrate on analytics, decision support, and connectivity to electronic health records to enhance clinical utility. Consequently, cross-sector collaboration between instrument manufacturers, reagent suppliers, and software providers has become a distinguishing feature of successful market entrants.In addition, companies are aligning product roadmaps with clinical needs such as rapid pathogen identification, longitudinal monitoring, and minimally invasive diagnostics. Strategic transactions and co-development agreements are common as organizations seek to accelerate time-to-market and fill functional gaps. Importantly, regulatory compliance and real-world evidence generation increasingly determine market access, prompting firms to expand post-market surveillance and generate clinical data that substantiate claimed benefits. Firms that combine technical excellence with robust distribution channels, clinical validation, and responsive customer support are most likely to sustain competitive advantage
Actionable strategic guidance for executives to strengthen supply chain resilience, accelerate technology adoption, and align commercial models with clinical realities
Industry leaders should pursue an integrated strategy that balances near-term operational resilience with long-term innovation and market access. First, organizations must diversify supply chains and qualify geographically distributed suppliers for critical inputs to mitigate tariff exposure and logistical disruptions. Second, investments in modular platforms and reagent-flexible assays will reduce vendor lock-in and enable rapid adaptation to changing disease profiles. Third, firms should prioritize software and data capabilities that enable workflow automation, clinical decision support, and interoperability with electronic health records to increase adoption across hospitals, laboratories, and home care settings.Furthermore, proactive regulatory engagement and generation of pragmatic clinical evidence will accelerate reimbursement conversations and adoption. Leaders should explore partnership models, including reagent rental, outcome-linked contracting, and co-marketing arrangements, to lower adoption barriers for customers. Finally, workforce development and customer support programs that simplify implementation, ensure quality, and demonstrate economic value will facilitate sustained uptake and improve patient outcomes
Transparent explanation of the mixed-methods research design, data triangulation, and segmentation rationale that underpin the report’s conclusions and insights
The research approach underpinning this analysis integrates qualitative and quantitative methods to produce rigorous, actionable findings. Primary inputs include structured interviews with clinical laboratory directors, hospital procurement officers, diagnostic developers, and policy experts to capture operational realities and strategic intent. Secondary sources encompass peer-reviewed literature, regulatory guidance, and publicly available technical documentation to validate technology attributes and clinical use cases. Data triangulation and expert validation were used to reconcile divergent perspectives and to stress-test key assumptions.Segmentation frameworks were constructed to mirror clinical workflows and procurement behavior, encompassing test types, product components, age cohorts, technologies, test purposes, disease categories, and end-user settings. Regional analysis employed policy and infrastructure indicators to identify adoption drivers and constraints. Limitations include variability in reporting standards across jurisdictions and the evolving nature of regulatory guidance, which can affect comparability; to address this, sensitivity checks and scenario-based analysis were applied. Ethical considerations guided the treatment of proprietary information and ensured that stakeholder perspectives were anonymized and contextualized
Concise synthesis of the strategic imperatives and operational priorities that will determine which organizations succeed in the evolving respiratory diagnostics landscape
In summary, respiratory disease testing is experiencing a period of meaningful transformation driven by technological innovation, changing care delivery models, and evolving policy environments. Molecular diagnostics, advanced imaging, and digital health solutions are converging to support more timely diagnosis, tailored monitoring, and improved prognostication across diverse patient populations. At the same time, supply chain pressures and trade policy shifts have introduced complexity that requires strategic sourcing and adaptive commercial models.Consequently, organizations that combine technical rigor with operational flexibility, evidence generation, and customer-centric implementation will be best positioned to deliver clinical impact and commercial success. Collaboration across manufacturers, laboratories, providers, and payers will be essential to translate technological capability into accessible and sustainable diagnostic services. Ultimately, a balanced emphasis on innovation, quality, and pragmatic execution will determine which stakeholders lead the next phase of respiratory care
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
19. China Respiratory Disease Testing Market
Companies Mentioned
The key companies profiled in this Respiratory Disease Testing market report include:- Abbott Laboratories
- AstraZeneca PLC
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Biomérieux PLC
- Charles River Laboratories
- COSMED srl
- Eurofins Viracor, LLC
- F. Hoffmann-La Roche Ltd.
- Fujifilm Holdings
- GE Healthcare
- Koninklijke Philips N.V
- Medtronic PLC
- MGC Diagnostics Corporation by Caire Inc.
- QIAGEN Group
- ResMed
- SDI Diagnostics, Inc.
- Seegene Inc.
- Seimens Healthineers AG
- Thermo Fisher Scientific
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
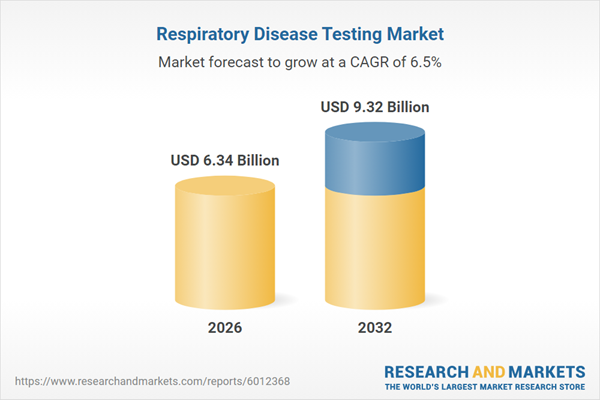
| Estimated Market Value ( USD | $ 6.34 Billion |
| Forecasted Market Value ( USD | $ 9.32 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |