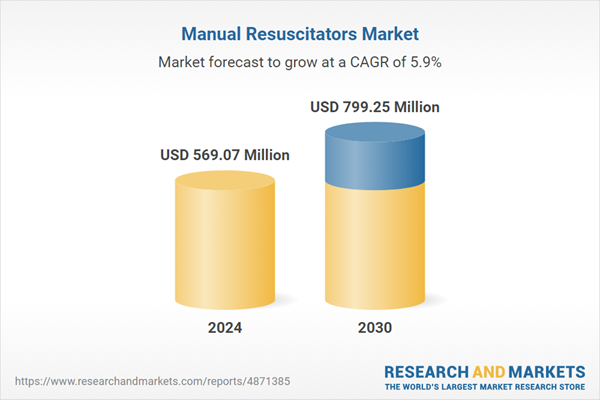
The Manual Resuscitators Market was valued at USD 569.07 million in 2024, and is projected to reach USD 799.25 million by 2030, rising at a CAGR of 5.90%. The demand for manual resuscitators/ Bag Valve Mask (BVM) is expected to grow owing to the rising cases of COPD and the increasing need for neonatal ventilation.
Moreover, the increasing obese population is likely to increase the risk of cardiac arrest cases, in turn driving the demand for artificial ventilation. According to National Health and Nutrition Examination Survey (NHANES) data of 2017-2018, 2 in 5 adults suffer from obesity, which accounts for almost 44% of the U.S. population. According to World Health Organization (WHO), in 2020, around 39 million children suffered from obesity under the age of 5.
The rising mortality due to non-communicable diseases, such as coronary heart disease has created a pressing need to increase awareness about the measures to be taken to help a patient. Countries such as Germany, the U.K., and France are undertaking initiatives to create awareness amongst people about CPR and heart problems. According to the World Health Organization (WHO), approximately 41 million people die every year due to non-communicable diseases. Cardiovascular disease accounts for around 17.9 million deaths every year. This is projected to bode well with the market.
To address the growing death rate from cardiac arrest, organizations such as the American Heart Association, Red Cross, and Resuscitation Council (U.K., Australia, and Europe), also assist the countries to increase the survival rates of sudden cardiac arrest. These activities include providing training to perform artificial ventilation, providing resuscitation kits, and encouraging bystanders to help patients in emergency cases.
Moreover, the COVID-19 pandemic has positively impacted the market owing to the huge requirement of oxygen and owing to the lack of supply of ventilators. Manual resuscitators were in huge demand as a portable replacement for the ventilators. For instance, the demand for Ambu’s resuscitators and single-use scopes was greater during the pandemic. Ambu’s witnessed 21% organic revenue growth in the Q3 of 2020.
This product will be delivered within 1-3 business days.
Moreover, the increasing obese population is likely to increase the risk of cardiac arrest cases, in turn driving the demand for artificial ventilation. According to National Health and Nutrition Examination Survey (NHANES) data of 2017-2018, 2 in 5 adults suffer from obesity, which accounts for almost 44% of the U.S. population. According to World Health Organization (WHO), in 2020, around 39 million children suffered from obesity under the age of 5.
The rising mortality due to non-communicable diseases, such as coronary heart disease has created a pressing need to increase awareness about the measures to be taken to help a patient. Countries such as Germany, the U.K., and France are undertaking initiatives to create awareness amongst people about CPR and heart problems. According to the World Health Organization (WHO), approximately 41 million people die every year due to non-communicable diseases. Cardiovascular disease accounts for around 17.9 million deaths every year. This is projected to bode well with the market.
To address the growing death rate from cardiac arrest, organizations such as the American Heart Association, Red Cross, and Resuscitation Council (U.K., Australia, and Europe), also assist the countries to increase the survival rates of sudden cardiac arrest. These activities include providing training to perform artificial ventilation, providing resuscitation kits, and encouraging bystanders to help patients in emergency cases.
Moreover, the COVID-19 pandemic has positively impacted the market owing to the huge requirement of oxygen and owing to the lack of supply of ventilators. Manual resuscitators were in huge demand as a portable replacement for the ventilators. For instance, the demand for Ambu’s resuscitators and single-use scopes was greater during the pandemic. Ambu’s witnessed 21% organic revenue growth in the Q3 of 2020.
Manual Resuscitators Market Report Highlights
- Based on type, the self-inflating segment accounted for the maximum market share in 2024 as they have greater usability in emergency situations, where bystander cardiopulmonary resuscitation is necessary. Companies are striving to launch newer integrated products, which can help in overcoming the delayed ventilation issues.
- The flow-inflating segment also referred to as anesthesia bags is expected to witness a rapid rise in demand since they are majorly used in ICUs, where 100% oxygen delivery is of utmost importance. The increasing birth rate and increasing efforts by global organizations such as UNICEFs to curb neonatal deaths at the time of birth in low-resource nations are further expected to propel the segment growth in the coming years.
- In terms of end-use, the hospital segment dominated the market in 2024 due to the requirement for artificial ventilation in the delivery and neonatal ward. With the increasing admission of cardiac arrest patients, the need for resuscitators is expected to further increase.
- There has been a global increase in the number of out-of-hospital cardiac arrests globally. Each year about 326,000 cases of cardiac arrest occur outside the hospital setting which accounts for the major cause of death in the U.S. and around 200,000 cardiac arrests occur in hospitals. This is contributing to the expansion of the out-of-hospital segment.
- North America dominated the market in 2024. The introduction of state-of-the-art resuscitation products and training kits to reduce the chances of delayed ventilation are the major drivers of the market.
This report addresses:
- Market intelligence to enable effective decision-making.
- Market estimates and forecasts from 2018 to 2030.
- Growth opportunities and trend analyses.
- Segment and regional revenue forecasts for market assessment.
- Competition strategy and market share analysis.
- Product innovation listings for you to stay ahead of the curve.
- COVID-19's impact and how to sustain in this fast-evolving market.
Why Should You Buy This Report?
- Comprehensive Market Analysis: Gain detailed insights into the market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This product will be delivered within 1-3 business days.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation and Scope
1.2. Market Definitions
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased Database
1.4.2. Internal Database
1.5. Details of primary research
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.1.1. Approach 1: Commodity flow approach
1.7.1.2. Approach 2: Volume price analysis
1.8. Research Scope and Assumptions
1.8.1. List of Secondary Sources
1.8.2. List of Primary Sources
1.8.3. Objectives
1.2. Market Definitions
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased Database
1.4.2. Internal Database
1.5. Details of primary research
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.1.1. Approach 1: Commodity flow approach
1.7.1.2. Approach 2: Volume price analysis
1.8. Research Scope and Assumptions
1.8.1. List of Secondary Sources
1.8.2. List of Primary Sources
1.8.3. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.2.1. Type Segment Outlook
2.2.2. Modality Segment Outlook
2.2.3. Material Segment Outlook
2.2.4. Technology Segment Outlook
2.2.5. Patient Type Segment Outlook
2.2.6. Application Segment Outlook
2.2.7. End Use Segment Outlook
2.2.8. Regional Outlook
2.3. Competitive Insights
2.2. Segment Outlook
2.2.1. Type Segment Outlook
2.2.2. Modality Segment Outlook
2.2.3. Material Segment Outlook
2.2.4. Technology Segment Outlook
2.2.5. Patient Type Segment Outlook
2.2.6. Application Segment Outlook
2.2.7. End Use Segment Outlook
2.2.8. Regional Outlook
2.3. Competitive Insights
Chapter 3. Manual Resuscitators Market Variables, Trends, & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Industry Analysis
3.2.1. User Perspective Analysis
3.2.2. Key End Users
3.3. Market Dynamics
3.3.1. Market Drivers Analysis
3.3.1.1. Increasing prevalence of respiratory diseases
3.3.1.2. Growing Advancements in Healthcare Infrastructure
3.3.1.3. Rising healthcare awareness
3.3.2. Market Restraints Analysis
3.3.2.1. Increasing safety concerns
3.3.2.2. Availability of alternative respiratory support devices
3.3.3. Industry Challenges and Opportunity Analysis
3.4. Manual Resuscitators Market Analysis Tools
3.4.1. Porter’s Analysis
3.4.1.1. Bargaining power of the suppliers
3.4.1.2. Bargaining power of the buyers
3.4.1.3. Threats of substitution
3.4.1.4. Threats from new entrants
3.4.1.5. Competitive rivalry
3.4.2. PESTEL Analysis
3.4.2.1. Political landscape
3.4.2.2. Economic and Social landscape
3.4.2.3. Technological landscape
3.4.2.4. Environmental landscape
3.4.2.5. Legal Landscape
3.5. Regulatory Framework
3.6. Technology Outlook
3.7. Impact of COVID-19
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Industry Analysis
3.2.1. User Perspective Analysis
3.2.2. Key End Users
3.3. Market Dynamics
3.3.1. Market Drivers Analysis
3.3.1.1. Increasing prevalence of respiratory diseases
3.3.1.2. Growing Advancements in Healthcare Infrastructure
3.3.1.3. Rising healthcare awareness
3.3.2. Market Restraints Analysis
3.3.2.1. Increasing safety concerns
3.3.2.2. Availability of alternative respiratory support devices
3.3.3. Industry Challenges and Opportunity Analysis
3.4. Manual Resuscitators Market Analysis Tools
3.4.1. Porter’s Analysis
3.4.1.1. Bargaining power of the suppliers
3.4.1.2. Bargaining power of the buyers
3.4.1.3. Threats of substitution
3.4.1.4. Threats from new entrants
3.4.1.5. Competitive rivalry
3.4.2. PESTEL Analysis
3.4.2.1. Political landscape
3.4.2.2. Economic and Social landscape
3.4.2.3. Technological landscape
3.4.2.4. Environmental landscape
3.4.2.5. Legal Landscape
3.5. Regulatory Framework
3.6. Technology Outlook
3.7. Impact of COVID-19
Chapter 4. Manual Resuscitators Market: Type Estimates & Trend Analysis
4.1. Manual Resuscitators Market: Type Movement Analysis
4.2. Manual Resuscitators Market: Type Segment Dashboard
4.3. Type Movement & Market Share Analysis, 2024 & 2030
4.4. Manual Resuscitators Market Estimates & Forecast, by Type
4.5. Self-Inflating
4.5.1. Self-inflating market, 2018-2030 (USD Million)
4.6. Flow Inflating
4.6.1. Flow inflating market, 2018-2030 (USD Million)
4.7. T-piece
4.7.1. T-piece market, 2018-2030 (USD Million)
4.2. Manual Resuscitators Market: Type Segment Dashboard
4.3. Type Movement & Market Share Analysis, 2024 & 2030
4.4. Manual Resuscitators Market Estimates & Forecast, by Type
4.5. Self-Inflating
4.5.1. Self-inflating market, 2018-2030 (USD Million)
4.6. Flow Inflating
4.6.1. Flow inflating market, 2018-2030 (USD Million)
4.7. T-piece
4.7.1. T-piece market, 2018-2030 (USD Million)
Chapter 5. Manual Resuscitators Market: Modality Estimates & Trend Analysis
5.1. Manual Resuscitators Market: Modality Movement Analysis
5.2. Manual Resuscitators Market: Modality Segment Dashboard
5.3. Modality Movement & Market Share Analysis, 2024 & 2030
5.4. Manual Resuscitators Market Estimates & Forecast, by Modality
5.5. Disposable
5.5.1. Disposable market, 2018-2030 (USD Million)
5.6. Reusable
5.6.1. Reusable market, 2018-2030 (USD Million)
5.2. Manual Resuscitators Market: Modality Segment Dashboard
5.3. Modality Movement & Market Share Analysis, 2024 & 2030
5.4. Manual Resuscitators Market Estimates & Forecast, by Modality
5.5. Disposable
5.5.1. Disposable market, 2018-2030 (USD Million)
5.6. Reusable
5.6.1. Reusable market, 2018-2030 (USD Million)
Chapter 6. Manual Resuscitators Market: Material Estimates & Trend Analysis
6.1. Manual Resuscitators Market: Material Movement Analysis
6.2. Manual Resuscitators Market: Material Segment Dashboard
6.3. Material Movement & Market Share Analysis, 2024 & 2030
6.4. Manual Resuscitators Market Estimates & Forecast, by Material
6.5. Silicon
6.5.1. Silicon market, 2018-2030 (USD Million)
6.6. PVC
6.6.1. PVC market, 2018-2030 (USD Million)
6.7. Rubber
6.7.1. Rubber market, 2018-2030 (USD Million)
6.2. Manual Resuscitators Market: Material Segment Dashboard
6.3. Material Movement & Market Share Analysis, 2024 & 2030
6.4. Manual Resuscitators Market Estimates & Forecast, by Material
6.5. Silicon
6.5.1. Silicon market, 2018-2030 (USD Million)
6.6. PVC
6.6.1. PVC market, 2018-2030 (USD Million)
6.7. Rubber
6.7.1. Rubber market, 2018-2030 (USD Million)
Chapter 7. Manual Resuscitators Market: Technology Estimates & Trend Analysis
7.1. Manual Resuscitators Market: Technology Movement Analysis
7.2. Manual Resuscitators Market: Technology Segment Dashboard
7.3. Technology Movement & Market Share Analysis, 2024 & 2030
7.4. Manual Resuscitators Market Estimates & Forecast, by Technology
7.5. Pop-off Valve
7.5.1. Pop-off valve market, 2018-2030 (USD Million)
7.6. PEEP Valve
7.6.1. PEEP valve market, 2018-2030 (USD Million)
7.7. Others
7.7.1. Others market, 2018-2030 (USD Million)
7.2. Manual Resuscitators Market: Technology Segment Dashboard
7.3. Technology Movement & Market Share Analysis, 2024 & 2030
7.4. Manual Resuscitators Market Estimates & Forecast, by Technology
7.5. Pop-off Valve
7.5.1. Pop-off valve market, 2018-2030 (USD Million)
7.6. PEEP Valve
7.6.1. PEEP valve market, 2018-2030 (USD Million)
7.7. Others
7.7.1. Others market, 2018-2030 (USD Million)
Chapter 8. Manual Resuscitators Market: Patient Type Estimates & Trend Analysis
8.1. Manual Resuscitators Market: Patient Type Movement Analysis
8.2. Manual Resuscitators Market: Patient Type Segment Dashboard
8.3. Patient Type Movement & Market Share Analysis, 2024 & 2030
8.4. Manual Resuscitators Market Estimates & Forecast, by Patient Type
8.5. Adult
8.5.1. Adult market, 2018-2030 (USD Million)
8.6. Pediatric
8.6.1. Pediatric market, 2018-2030 (USD Million)
8.7. Others
8.7.1. Others market, 2018-2030 (USD Million)
8.2. Manual Resuscitators Market: Patient Type Segment Dashboard
8.3. Patient Type Movement & Market Share Analysis, 2024 & 2030
8.4. Manual Resuscitators Market Estimates & Forecast, by Patient Type
8.5. Adult
8.5.1. Adult market, 2018-2030 (USD Million)
8.6. Pediatric
8.6.1. Pediatric market, 2018-2030 (USD Million)
8.7. Others
8.7.1. Others market, 2018-2030 (USD Million)
Chapter 9. Manual Resuscitators Market: Application Estimates & Trend Analysis
9.1. Manual Resuscitators Market: Application Analysis
9.2. Manual Resuscitators Market: Application Segment Dashboard
9.3. Application Movement & Market Share Analysis, 2024 & 2030
9.4. Manual Resuscitators Market Estimates & Forecast, by Application
9.5. Chronic Obstructive Pulmonary Disease
9.5.1. Chronic obstructive pulmonary disease market, 2018-2030 (USD Million)
9.6. Cardiopulmonary Arrest
9.6.1. Cardiopulmonary arrest market, 2018-2030 (USD Million)
9.7. Others
9.7.1. Others market, 2018-2030 (USD Million)
9.2. Manual Resuscitators Market: Application Segment Dashboard
9.3. Application Movement & Market Share Analysis, 2024 & 2030
9.4. Manual Resuscitators Market Estimates & Forecast, by Application
9.5. Chronic Obstructive Pulmonary Disease
9.5.1. Chronic obstructive pulmonary disease market, 2018-2030 (USD Million)
9.6. Cardiopulmonary Arrest
9.6.1. Cardiopulmonary arrest market, 2018-2030 (USD Million)
9.7. Others
9.7.1. Others market, 2018-2030 (USD Million)
Chapter 10. Manual Resuscitators Market: End Use Estimates & Trend Analysis
10.1. Manual Resuscitators Market: End Use Movement Analysis
10.2. Manual Resuscitators Market: End Use Segment Dashboard
10.3. End Use Movement & Market Share Analysis, 2024 & 2030
10.4. Manual Resuscitators Market Estimates & Forecast, by End Use
10.5. Hospital
10.5.1. Hospital market, 2018-2030 (USD Million)
10.6. Out-of-hospital
10.6.1. Out-of-hospital market, 2018-2030 (USD Million)
10.7. ICUs
10.7.1. ICUs market, 2018-2030 (USD Million)
10.8. ASC
10.8.1. ASC market, 2018-2030 (USD Million)
10.9. Military
10.9.1. Military market, 2018-2030 (USD Million)
10.10. Others
10.10.1. Others market, 2018-2030 (USD Million)
10.2. Manual Resuscitators Market: End Use Segment Dashboard
10.3. End Use Movement & Market Share Analysis, 2024 & 2030
10.4. Manual Resuscitators Market Estimates & Forecast, by End Use
10.5. Hospital
10.5.1. Hospital market, 2018-2030 (USD Million)
10.6. Out-of-hospital
10.6.1. Out-of-hospital market, 2018-2030 (USD Million)
10.7. ICUs
10.7.1. ICUs market, 2018-2030 (USD Million)
10.8. ASC
10.8.1. ASC market, 2018-2030 (USD Million)
10.9. Military
10.9.1. Military market, 2018-2030 (USD Million)
10.10. Others
10.10.1. Others market, 2018-2030 (USD Million)
Chapter 11. Manual Resuscitators Market: Regional Estimates & Trend Analysis by Region, Type, Modality, Material, Technology, Patient Type, Application, End Use
11.1. Regional Market Snapshot
11.2. Regional Market Share Analysis, 2024 & 2030
11.3. SWOT Analysis
11.3.1. North America
11.3.2. Europe
11.3.3. Asia Pacific
11.3.4. Latin America
11.3.5. Middle East & Africa
11.4. Manual Resuscitators Market Share, by Region, 2024 & 2030, USD Million
11.5. North America
11.5.1. North America Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.5.2. U.S.
11.5.2.1. Key Country Dynamics
11.5.2.2. Regulatory Landscape/Reimbursement Scenario
11.5.2.3. Competitive Insights
11.5.2.4. Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.5.3. Canada
11.5.3.1. Key Country Dynamics
11.5.3.2. Regulatory Landscape/Reimbursement Scenario
11.5.3.3. Competitive Insights
11.5.3.4. Canada Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.5.4. Mexico
11.5.4.1. Key Country Dynamics
11.5.4.2. Regulatory Landscape/Reimbursement Scenario
11.5.4.3. Competitive Insights
11.5.4.4. Canada Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6. Europe
11.6.1. Europe Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.2. UK
11.6.2.1. Key Country Dynamics
11.6.2.2. Regulatory Landscape/Reimbursement Scenario
11.6.2.3. Competitive Insights
11.6.2.4. UK Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.3. Germany
11.6.3.1. Key Country Dynamics
11.6.3.2. Regulatory Landscape/Reimbursement Scenario
11.6.3.3. Competitive Insights
11.6.3.4. Germany Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.4. France
11.6.4.1. Key Country Dynamics
11.6.4.2. Regulatory Landscape/Reimbursement Scenario
11.6.4.3. Competitive Insights
11.6.4.4. France Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.5. Italy
11.6.5.1. Key Country Dynamics
11.6.5.2. Regulatory Landscape/Reimbursement Scenario
11.6.5.3. Competitive Insights
11.6.5.4. Italy Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.6. Spain
11.6.6.1. Key Country Dynamics
11.6.6.2. Regulatory Landscape/Reimbursement Scenario
11.6.6.3. Competitive Insights
11.6.6.4. Spain Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.7. Sweden
11.6.7.1. Key Country Dynamics
11.6.7.2. Regulatory Landscape/Reimbursement Scenario
11.6.7.3. Competitive Insights
11.6.7.4. Sweden Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.8. Denmark
11.6.8.1. Key Country Dynamics
11.6.8.2. Regulatory Landscape/Reimbursement Scenario
11.6.8.3. Competitive Insights
11.6.8.4. Sweden Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.9. Norway
11.6.9.1. Key Country Dynamics
11.6.9.2. Regulatory Landscape/Reimbursement Scenario
11.6.9.3. Competitive Insights
11.6.9.4. Sweden Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7. Asia Pacific
11.7.1. Asia Pacific Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.2. China
11.7.2.1. Key Country Dynamics
11.7.2.2. Regulatory Landscape/Reimbursement Scenario
11.7.2.3. Competitive Insights
11.7.2.4. China Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.3. Japan
11.7.3.1. Key Country Dynamics
11.7.3.2. Regulatory Landscape/Reimbursement Scenario
11.7.3.3. Competitive Insights
11.7.3.4. Japan Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.4. India
11.7.4.1. Key Country Dynamics
11.7.4.2. Regulatory Landscape/Reimbursement Scenario
11.7.4.3. Competitive Insights
11.7.4.4. India Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.5. South Korea
11.7.5.1. Key Country Dynamics
11.7.5.2. Regulatory Landscape/Reimbursement Scenario
11.7.5.3. Competitive Insights
11.7.5.4. South Korea Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.6. Australia
11.7.6.1. Key Country Dynamics
11.7.6.2. Regulatory Landscape/Reimbursement Scenario
11.7.6.3. Competitive Insights
11.7.6.4. Australia Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.7. Thailand
11.7.7.1. Key Country Dynamics
11.7.7.2. Regulatory Landscape/Reimbursement Scenario
11.7.7.3. Competitive Insights
11.7.7.4. Thailand Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.8. Latin America
11.8.1. Latin America Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.8.2. Brazil
11.8.2.1. Key Country Dynamics
11.8.2.2. Regulatory Landscape/Reimbursement Scenario
11.8.2.3. Competitive Insights
11.8.2.4. Brazil Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.8.3. Argentina
11.8.3.1. Key Country Dynamics
11.8.3.2. Regulatory Landscape/Reimbursement Scenario
11.8.3.3. Competitive Insights
11.8.3.4. Argentina Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9. Middle East and Africa
11.9.1. Middle East and Africa Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9.2. South Africa
11.9.2.1. Key Country Dynamics
11.9.2.2. Regulatory Landscape/Reimbursement Scenario
11.9.2.3. Competitive Insights
11.9.2.4. South Africa Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9.3. Saudi Arabia
11.9.3.1. Key Country Dynamics
11.9.3.2. Regulatory Landscape/Reimbursement Scenario
11.9.3.3. Competitive Insights
11.9.3.4. Saudi Arabia Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9.4. UAE
11.9.4.1. Key Country Dynamics
11.9.4.2. Regulatory Landscape/Reimbursement Scenario
11.9.4.3. Competitive Insights
11.9.4.4. UAE Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9.5. Kuwait
11.9.5.1. Key Country Dynamics
11.9.5.2. Regulatory Landscape/Reimbursement Scenario
11.9.5.3. Competitive Insights
11.9.5.4. Kuwait Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.2. Regional Market Share Analysis, 2024 & 2030
11.3. SWOT Analysis
11.3.1. North America
11.3.2. Europe
11.3.3. Asia Pacific
11.3.4. Latin America
11.3.5. Middle East & Africa
11.4. Manual Resuscitators Market Share, by Region, 2024 & 2030, USD Million
11.5. North America
11.5.1. North America Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.5.2. U.S.
11.5.2.1. Key Country Dynamics
11.5.2.2. Regulatory Landscape/Reimbursement Scenario
11.5.2.3. Competitive Insights
11.5.2.4. Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.5.3. Canada
11.5.3.1. Key Country Dynamics
11.5.3.2. Regulatory Landscape/Reimbursement Scenario
11.5.3.3. Competitive Insights
11.5.3.4. Canada Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.5.4. Mexico
11.5.4.1. Key Country Dynamics
11.5.4.2. Regulatory Landscape/Reimbursement Scenario
11.5.4.3. Competitive Insights
11.5.4.4. Canada Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6. Europe
11.6.1. Europe Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.2. UK
11.6.2.1. Key Country Dynamics
11.6.2.2. Regulatory Landscape/Reimbursement Scenario
11.6.2.3. Competitive Insights
11.6.2.4. UK Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.3. Germany
11.6.3.1. Key Country Dynamics
11.6.3.2. Regulatory Landscape/Reimbursement Scenario
11.6.3.3. Competitive Insights
11.6.3.4. Germany Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.4. France
11.6.4.1. Key Country Dynamics
11.6.4.2. Regulatory Landscape/Reimbursement Scenario
11.6.4.3. Competitive Insights
11.6.4.4. France Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.5. Italy
11.6.5.1. Key Country Dynamics
11.6.5.2. Regulatory Landscape/Reimbursement Scenario
11.6.5.3. Competitive Insights
11.6.5.4. Italy Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.6. Spain
11.6.6.1. Key Country Dynamics
11.6.6.2. Regulatory Landscape/Reimbursement Scenario
11.6.6.3. Competitive Insights
11.6.6.4. Spain Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.7. Sweden
11.6.7.1. Key Country Dynamics
11.6.7.2. Regulatory Landscape/Reimbursement Scenario
11.6.7.3. Competitive Insights
11.6.7.4. Sweden Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.8. Denmark
11.6.8.1. Key Country Dynamics
11.6.8.2. Regulatory Landscape/Reimbursement Scenario
11.6.8.3. Competitive Insights
11.6.8.4. Sweden Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.6.9. Norway
11.6.9.1. Key Country Dynamics
11.6.9.2. Regulatory Landscape/Reimbursement Scenario
11.6.9.3. Competitive Insights
11.6.9.4. Sweden Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7. Asia Pacific
11.7.1. Asia Pacific Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.2. China
11.7.2.1. Key Country Dynamics
11.7.2.2. Regulatory Landscape/Reimbursement Scenario
11.7.2.3. Competitive Insights
11.7.2.4. China Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.3. Japan
11.7.3.1. Key Country Dynamics
11.7.3.2. Regulatory Landscape/Reimbursement Scenario
11.7.3.3. Competitive Insights
11.7.3.4. Japan Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.4. India
11.7.4.1. Key Country Dynamics
11.7.4.2. Regulatory Landscape/Reimbursement Scenario
11.7.4.3. Competitive Insights
11.7.4.4. India Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.5. South Korea
11.7.5.1. Key Country Dynamics
11.7.5.2. Regulatory Landscape/Reimbursement Scenario
11.7.5.3. Competitive Insights
11.7.5.4. South Korea Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.6. Australia
11.7.6.1. Key Country Dynamics
11.7.6.2. Regulatory Landscape/Reimbursement Scenario
11.7.6.3. Competitive Insights
11.7.6.4. Australia Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.7.7. Thailand
11.7.7.1. Key Country Dynamics
11.7.7.2. Regulatory Landscape/Reimbursement Scenario
11.7.7.3. Competitive Insights
11.7.7.4. Thailand Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.8. Latin America
11.8.1. Latin America Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.8.2. Brazil
11.8.2.1. Key Country Dynamics
11.8.2.2. Regulatory Landscape/Reimbursement Scenario
11.8.2.3. Competitive Insights
11.8.2.4. Brazil Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.8.3. Argentina
11.8.3.1. Key Country Dynamics
11.8.3.2. Regulatory Landscape/Reimbursement Scenario
11.8.3.3. Competitive Insights
11.8.3.4. Argentina Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9. Middle East and Africa
11.9.1. Middle East and Africa Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9.2. South Africa
11.9.2.1. Key Country Dynamics
11.9.2.2. Regulatory Landscape/Reimbursement Scenario
11.9.2.3. Competitive Insights
11.9.2.4. South Africa Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9.3. Saudi Arabia
11.9.3.1. Key Country Dynamics
11.9.3.2. Regulatory Landscape/Reimbursement Scenario
11.9.3.3. Competitive Insights
11.9.3.4. Saudi Arabia Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9.4. UAE
11.9.4.1. Key Country Dynamics
11.9.4.2. Regulatory Landscape/Reimbursement Scenario
11.9.4.3. Competitive Insights
11.9.4.4. UAE Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
11.9.5. Kuwait
11.9.5.1. Key Country Dynamics
11.9.5.2. Regulatory Landscape/Reimbursement Scenario
11.9.5.3. Competitive Insights
11.9.5.4. Kuwait Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Chapter 12. Competitive Landscape
12.1. Recent Developments & Impact Analysis by Key Market Participants
12.2. Company Categorization
12.3. Company Market Share Analysis
12.4. Company Heat Map Analysis
12.5. Company Profiles
12.5.1. WEINMANN Emergency Medical Technology GmbH + Co. KG
12.5.1.1. Participant’s Overview
12.5.1.2. Financial Performance
12.5.1.3. Product Benchmarking
12.5.1.4. Strategic Initiatives
12.5.2. Laerdal Medical
12.5.2.1. Participant’s Overview
12.5.2.2. Financial Performance
12.5.2.3. Product Benchmarking
12.5.2.4. Strategic Initiatives
12.5.3. Ambu A/S
12.5.3.1. Participant’s Overview
12.5.3.2. Financial Performance
12.5.3.3. Product Benchmarking
12.5.3.4. Strategic Initiatives
12.5.4. Medline Industries, LP
12.5.4.1. Participant’s Overview
12.5.4.2. Financial Performance
12.5.4.3. Product Benchmarking
12.5.4.4. Strategic Initiatives
12.5.5. VYAIRE
12.5.5.1. Participant’s Overview
12.5.5.2. Financial Performance
12.5.5.3. Product Benchmarking
12.5.5.4. Strategic Initiatives
12.5.6. ResMed
12.5.6.1. Participant’s Overview
12.5.6.2. Financial Performance
12.5.6.3. Product Benchmarking
12.5.6.4. Strategic Initiatives
12.5.7. HUM GmbH
12.5.7.1. Participant’s Overview
12.5.7.2. Financial Performance
12.5.7.3. Product Benchmarking
12.5.7.4. Strategic Initiatives
12.5.8. CareFusion (Acquired by BD in 2014)
12.5.8.1. Participant’s Overview
12.5.8.2. Financial Performance
12.5.8.3. Product Benchmarking
12.5.8.4. Strategic Initiatives
12.5.9. Medtronic Plc
12.5.9.1. Participant’s Overview
12.5.9.2. Financial Performance
12.5.9.3. Product Benchmarking
12.5.9.4. Strategic Initiatives
12.5.10. Teleflex Inc.
12.5.10.1. Participant’s Overview
12.5.10.2. Financial Performance
12.5.10.3. Product Benchmarking
12.5.10.4. Strategic Initiatives
12.5.11. Hopkins Medical Products (marketlab)
12.5.11.1. Participant’s Overview
12.5.11.2. Financial Performance
12.5.11.3. Product Benchmarking
12.5.11.4. Strategic Initiatives
12.5.12. PerSys Medical (Safeguard)
12.5.12.1. Participant’s Overview
12.5.12.2. Financial Performance
12.5.12.3. Product Benchmarking
12.5.12.4. Strategic Initiatives
12.2. Company Categorization
12.3. Company Market Share Analysis
12.4. Company Heat Map Analysis
12.5. Company Profiles
12.5.1. WEINMANN Emergency Medical Technology GmbH + Co. KG
12.5.1.1. Participant’s Overview
12.5.1.2. Financial Performance
12.5.1.3. Product Benchmarking
12.5.1.4. Strategic Initiatives
12.5.2. Laerdal Medical
12.5.2.1. Participant’s Overview
12.5.2.2. Financial Performance
12.5.2.3. Product Benchmarking
12.5.2.4. Strategic Initiatives
12.5.3. Ambu A/S
12.5.3.1. Participant’s Overview
12.5.3.2. Financial Performance
12.5.3.3. Product Benchmarking
12.5.3.4. Strategic Initiatives
12.5.4. Medline Industries, LP
12.5.4.1. Participant’s Overview
12.5.4.2. Financial Performance
12.5.4.3. Product Benchmarking
12.5.4.4. Strategic Initiatives
12.5.5. VYAIRE
12.5.5.1. Participant’s Overview
12.5.5.2. Financial Performance
12.5.5.3. Product Benchmarking
12.5.5.4. Strategic Initiatives
12.5.6. ResMed
12.5.6.1. Participant’s Overview
12.5.6.2. Financial Performance
12.5.6.3. Product Benchmarking
12.5.6.4. Strategic Initiatives
12.5.7. HUM GmbH
12.5.7.1. Participant’s Overview
12.5.7.2. Financial Performance
12.5.7.3. Product Benchmarking
12.5.7.4. Strategic Initiatives
12.5.8. CareFusion (Acquired by BD in 2014)
12.5.8.1. Participant’s Overview
12.5.8.2. Financial Performance
12.5.8.3. Product Benchmarking
12.5.8.4. Strategic Initiatives
12.5.9. Medtronic Plc
12.5.9.1. Participant’s Overview
12.5.9.2. Financial Performance
12.5.9.3. Product Benchmarking
12.5.9.4. Strategic Initiatives
12.5.10. Teleflex Inc.
12.5.10.1. Participant’s Overview
12.5.10.2. Financial Performance
12.5.10.3. Product Benchmarking
12.5.10.4. Strategic Initiatives
12.5.11. Hopkins Medical Products (marketlab)
12.5.11.1. Participant’s Overview
12.5.11.2. Financial Performance
12.5.11.3. Product Benchmarking
12.5.11.4. Strategic Initiatives
12.5.12. PerSys Medical (Safeguard)
12.5.12.1. Participant’s Overview
12.5.12.2. Financial Performance
12.5.12.3. Product Benchmarking
12.5.12.4. Strategic Initiatives
List of Tables
Table 1 List of secondary sources
Table 2 List of abbreviations
Table 3 North America manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 4 North America manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 5 North America manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 6 North America manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 7 North America manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 8 North America manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 9 North America manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 10 U.S. manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 11 U.S. manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 12 U.S. manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 13 U.S. manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 14 U.S. manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 15 U.S. manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 16 U.S. manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 17 Canada manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 18 Canada manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 19 Canada manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 20 Canada manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 21 Canada manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 22 Canada manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 23 Canada manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 24 Mexico manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 25 Mexico manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 26 Mexico manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 27 Mexico manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 28 Mexico manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 29 Mexico manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 30 Mexico manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 31 Europe manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 32 Europe manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 33 Europe manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 34 Europe manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 35 Europe manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 36 Europe manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 37 Europe manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 38 UK manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 39 UK manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 40 UK manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 41 UK manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 42 UK manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 43 UK manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 44 UK manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 45 Germany manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 46 Germany manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 47 Germany manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 48 Germany manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 49 Germany manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 50 Germany manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 51 Germany manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 52 France manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 53 France manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 54 France manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 55 France manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 56 France manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 57 France manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 58 France manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 59 Italy manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 60 Italy manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 61 Italy manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 62 Italy manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 63 Italy manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 64 Italy manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 65 Italy manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 66 Spain manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 67 Spain manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 68 Spain manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 69 Spain manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 70 Spain manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 71 Spain manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 72 Spain manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 73 Sweden manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 74 Sweden manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 75 Sweden manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 76 Sweden manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 77 Sweden manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 78 Sweden manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 79 Sweden manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 80 Denmark manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 81 Denmark manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 82 Denmark manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 83 Denmark manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 84 Denmark manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 85 Denmark manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 86 Denmark manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 87 Norway manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 88 Norway manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 89 Norway manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 90 Norway manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 91 Norway manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 92 Norway manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 93 Norway manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 94 Asia Pacific manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 95 Asia Pacific manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 96 Asia Pacific manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 97 Asia Pacific manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 98 Asia Pacific manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 99 Asia Pacific manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 100 Asia Pacific manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 101 Japan manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 102 Japan manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 103 Japan manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 104 Japan manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 105 Japan manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 106 Japan manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 107 Japan manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 108 China manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 109 China manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 110 China manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 111 China manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 112 China manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 113 North America manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 114 China manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 115 India manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 116 India manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 117 India manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 118 India manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 119 India manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 120 India manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 121 India manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 122 Australia manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 123 Australia manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 124 Australia manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 125 Australia manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 126 Australia manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 127 Australia manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 128 Australia manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 129 South Korea manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 130 South Korea manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 131 South Korea manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 132 South Korea manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 133 South Korea manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 134 South Korea manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 135 South Korea manual resuscitators market estimates and forecasts, by end use, 2018-2030 (USD Million)
Table 136 Thailand manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 137 Thailand manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 138 Thailand manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 139 Thailand manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 140 Thailand manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 141 Thailand manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 142 Thailand manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 143 Latin America manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 144 Latin America manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 145 Latin America manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 146 Latin America manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 147 Latin America manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 148 Latin America manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 149 Latin America manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 150 Brazil manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 151 Brazil manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 152 Brazil manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 153 Brazil manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 154 Brazil manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 155 Brazil manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 156 Brazil manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 157 Argentina manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 158 Argentina manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 159 Argentina manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 160 Argentina manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 161 Argentina manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 162 Argentina manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 163 Argentina manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 164 MEA manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 165 MEA manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 166 MEA manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 167 MEA manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 168 MEA manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 169 MEA manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 170 MEA manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 171 South Africa manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 172 South Africa manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 173 South Africa manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 174 South Africa manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 175 South Africa manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 176 South Africa manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 177 South Africa manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 178 Saudi Arabia manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 179 Saudi Arabia manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 180 Saudi Arabia manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 181 Saudi Arabia manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 182 Saudi Arabia manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 183 Saudi Arabia manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 184 Saudi Arabia manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 185 UAE manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 186 UAE manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 187 UAE manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 188 UAE manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 189 UAE manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 190 UAE manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 191 UAE manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 192 Kuwait manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 193 Kuwait manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 194 Kuwait manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 195 Kuwait manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 196 Kuwait manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 197 Kuwait manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 198 Kuwait manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 2 List of abbreviations
Table 3 North America manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 4 North America manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 5 North America manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 6 North America manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 7 North America manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 8 North America manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 9 North America manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 10 U.S. manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 11 U.S. manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 12 U.S. manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 13 U.S. manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 14 U.S. manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 15 U.S. manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 16 U.S. manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 17 Canada manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 18 Canada manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 19 Canada manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 20 Canada manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 21 Canada manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 22 Canada manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 23 Canada manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 24 Mexico manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 25 Mexico manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 26 Mexico manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 27 Mexico manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 28 Mexico manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 29 Mexico manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 30 Mexico manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 31 Europe manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 32 Europe manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 33 Europe manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 34 Europe manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 35 Europe manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 36 Europe manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 37 Europe manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 38 UK manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 39 UK manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 40 UK manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 41 UK manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 42 UK manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 43 UK manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 44 UK manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 45 Germany manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 46 Germany manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 47 Germany manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 48 Germany manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 49 Germany manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 50 Germany manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 51 Germany manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 52 France manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 53 France manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 54 France manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 55 France manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 56 France manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 57 France manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 58 France manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 59 Italy manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 60 Italy manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 61 Italy manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 62 Italy manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 63 Italy manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 64 Italy manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 65 Italy manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 66 Spain manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 67 Spain manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 68 Spain manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 69 Spain manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 70 Spain manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 71 Spain manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 72 Spain manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 73 Sweden manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 74 Sweden manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 75 Sweden manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 76 Sweden manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 77 Sweden manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 78 Sweden manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 79 Sweden manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 80 Denmark manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 81 Denmark manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 82 Denmark manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 83 Denmark manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 84 Denmark manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 85 Denmark manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 86 Denmark manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 87 Norway manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 88 Norway manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 89 Norway manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 90 Norway manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 91 Norway manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 92 Norway manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 93 Norway manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 94 Asia Pacific manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 95 Asia Pacific manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 96 Asia Pacific manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 97 Asia Pacific manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 98 Asia Pacific manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 99 Asia Pacific manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 100 Asia Pacific manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 101 Japan manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 102 Japan manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 103 Japan manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 104 Japan manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 105 Japan manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 106 Japan manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 107 Japan manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 108 China manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 109 China manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 110 China manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 111 China manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 112 China manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 113 North America manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 114 China manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 115 India manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 116 India manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 117 India manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 118 India manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 119 India manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 120 India manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 121 India manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 122 Australia manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 123 Australia manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 124 Australia manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 125 Australia manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 126 Australia manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 127 Australia manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 128 Australia manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 129 South Korea manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 130 South Korea manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 131 South Korea manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 132 South Korea manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 133 South Korea manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 134 South Korea manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 135 South Korea manual resuscitators market estimates and forecasts, by end use, 2018-2030 (USD Million)
Table 136 Thailand manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 137 Thailand manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 138 Thailand manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 139 Thailand manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 140 Thailand manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 141 Thailand manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 142 Thailand manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 143 Latin America manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 144 Latin America manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 145 Latin America manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 146 Latin America manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 147 Latin America manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 148 Latin America manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 149 Latin America manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 150 Brazil manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 151 Brazil manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 152 Brazil manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 153 Brazil manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 154 Brazil manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 155 Brazil manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 156 Brazil manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 157 Argentina manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 158 Argentina manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 159 Argentina manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 160 Argentina manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 161 Argentina manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 162 Argentina manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 163 Argentina manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 164 MEA manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 165 MEA manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 166 MEA manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 167 MEA manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 168 MEA manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 169 MEA manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 170 MEA manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 171 South Africa manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 172 South Africa manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 173 South Africa manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 174 South Africa manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 175 South Africa manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 176 South Africa manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 177 South Africa manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 178 Saudi Arabia manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 179 Saudi Arabia manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 180 Saudi Arabia manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 181 Saudi Arabia manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 182 Saudi Arabia manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 183 Saudi Arabia manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 184 Saudi Arabia manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 185 UAE manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 186 UAE manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 187 UAE manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 188 UAE manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 189 UAE manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 190 UAE manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 191 UAE manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
Table 192 Kuwait manual resuscitators market estimates and forecasts, by type, 2018-2030 (USD Million)
Table 193 Kuwait manual resuscitators market estimates and forecasts, by modality, 2018-2030 (USD Million)
Table 194 Kuwait manual resuscitators market estimates and forecasts, by material, 2018-2030 (USD Million)
Table 195 Kuwait manual resuscitators market estimates and forecasts by technology, 2018-2030 (USD Million)
Table 196 Kuwait manual resuscitators market estimates and forecasts by patient type, 2018-2030 (USD Million)
Table 197 Kuwait manual resuscitators market estimates and forecasts by application, 2018-2030 (USD Million)
Table 198 Kuwait manual resuscitators market estimates and forecasts by end use, 2018-2030 (USD Million)
List of Figures
Figure 1 List of Figures
Figure 2 Manual Resuscitators Market Segmentation
Figure 3 Information Procurement
Figure 4 Data Analysis Models
Figure 5 Market Formulation and Validation
Figure 6 Data Validating & Publishing
Figure 7 Market Snapshot
Figure 8 Segment Snapshot (1/2)
Figure 9 Segment Snapshot (2/2)
Figure 10 Competitive Landscape Snapshot
Figure 11 Manual Resuscitators - Market Size and Growth Prospects (USD Million)
Figure 12 Manual Resuscitators Market: Industry Value Chain Analysis
Figure 13 Manual Resuscitators Market: Market Dynamics
Figure 14 Manual Resuscitators Market: PORTER’s Analysis
Figure 15 Manual Resuscitators Market: PESTEL Analysis
Figure 16 Manual Resuscitators Market: Type segment dashboard
Figure 17 Manual Resuscitators Market: Type movement share analysis
Figure 18 Self-Inflating Market, 2018-2030 (USD Million)
Figure 19 Flow Inflating Market, 2018-2030 (USD Million)
Figure 20 T-piece Market, 2018-2030 (USD Million)
Figure 21 Manual Resuscitators Market: Modality segment dashboard
Figure 22 Manual Resuscitators Market: Modality Movement Share Analysis
Figure 23 Disposable Market, 2018-2030 (USD Million)
Figure 24 Reusable Market, 2018-2030 (USD Million)
Figure 25 Manual Resuscitators Market: Material segment dashboard
Figure 26 Manual Resuscitators Market: Material Movement Share Analysis
Figure 27 Silicon Market, 2018-2030 (USD Million)
Figure 28 PVC Market, 2018-2030 (USD Million)
Figure 29 Rubber Market, 2018-2030 (USD Million)
Figure 30 Manual Resuscitators Market: Technology segment dashboard
Figure 31 Manual Resuscitators Market: Technology Movement Share Analysis
Figure 32 Pop-off valve Market, 2018-2030 (USD Million)
Figure 33 PEEP valve Market, 2018-2030 (USD Million)
Figure 34 Others Market, 2018-2030 (USD Million)
Figure 35 Manual Resuscitators Market: Patient Type segment dashboard
Figure 36 Manual Resuscitators Market: Patient Type Movement Share Analysis
Figure 37 Adult Market, 2018-2030 (USD Million)
Figure 38 Pediatric Market, 2018-2030 (USD Million)
Figure 39 Others Market, 2018-2030 (USD Million)
Figure 40 Manual Resuscitators Market: Application segment dashboard
Figure 41 Manual Resuscitators Market: Application Movement Share Analysis
Figure 42 Chronic Obstructive Pulmonary Disease Market, 2018-2030 (USD Million)
Figure 43 Cardiopulmonary Arrest Market, 2018-2030 (USD Million)
Figure 44 Others Market, 2018-2030 (USD Million)
Figure 45 Manual Resuscitators Market: End Use Segment Dashboard
Figure 46 Manual Resuscitators Market: End Use Movement Share Analysis
Figure 47 Hospital Market, 2018-2030 (USD Million)
Figure 48 Out-of-hospital Market, 2018-2030 (USD Million)
Figure 49 ICUs Market, 2018-2030 (USD Million)
Figure 50 ASC Market, 2018-2030 (USD Million)
Figure 51 Military Market, 2018-2030 (USD Million)
Figure 52 Others (Alternate dispensing site, Closed door) Market, 2018-2030 (USD Million)
Figure 53 Manual Resuscitators Market Revenue, by Region, 2024 & 2030 (USD Million)
Figure 54 Regional Marketplace: Key Takeaways
Figure 55 North America Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 56 Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 57 Canada Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 58 Mexico Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 59 Europe Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 60 UK Manual Resuscitators Market Estimates and Forecasts, 2018-2030,) (USD Million)
Figure 61 Germany Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 62 France Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 63 Italy Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 64 Spain Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 65 Sweden Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 66 Denmark Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 67 Norway Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 68 Asia Pacific Manual Resuscitators Market Estimates and Forecast, 2018-2030 (USD Million)
Figure 69 Japan Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 70 China Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 71 India Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 72 Australia Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 73 Thailand Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 74 Latin America Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 75 Brazil Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 76 Argentina Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 77 MEA Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 78 Saudi Arabia Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 79 UAE Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 80 South Africa Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 81 Kuwait Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 82 Key Company Categorization
Figure 83 Company Market Positioning
Figure 84 Key Company Market Share Analysis, 2024
Figure 85 Strategic Framework
Figure 2 Manual Resuscitators Market Segmentation
Figure 3 Information Procurement
Figure 4 Data Analysis Models
Figure 5 Market Formulation and Validation
Figure 6 Data Validating & Publishing
Figure 7 Market Snapshot
Figure 8 Segment Snapshot (1/2)
Figure 9 Segment Snapshot (2/2)
Figure 10 Competitive Landscape Snapshot
Figure 11 Manual Resuscitators - Market Size and Growth Prospects (USD Million)
Figure 12 Manual Resuscitators Market: Industry Value Chain Analysis
Figure 13 Manual Resuscitators Market: Market Dynamics
Figure 14 Manual Resuscitators Market: PORTER’s Analysis
Figure 15 Manual Resuscitators Market: PESTEL Analysis
Figure 16 Manual Resuscitators Market: Type segment dashboard
Figure 17 Manual Resuscitators Market: Type movement share analysis
Figure 18 Self-Inflating Market, 2018-2030 (USD Million)
Figure 19 Flow Inflating Market, 2018-2030 (USD Million)
Figure 20 T-piece Market, 2018-2030 (USD Million)
Figure 21 Manual Resuscitators Market: Modality segment dashboard
Figure 22 Manual Resuscitators Market: Modality Movement Share Analysis
Figure 23 Disposable Market, 2018-2030 (USD Million)
Figure 24 Reusable Market, 2018-2030 (USD Million)
Figure 25 Manual Resuscitators Market: Material segment dashboard
Figure 26 Manual Resuscitators Market: Material Movement Share Analysis
Figure 27 Silicon Market, 2018-2030 (USD Million)
Figure 28 PVC Market, 2018-2030 (USD Million)
Figure 29 Rubber Market, 2018-2030 (USD Million)
Figure 30 Manual Resuscitators Market: Technology segment dashboard
Figure 31 Manual Resuscitators Market: Technology Movement Share Analysis
Figure 32 Pop-off valve Market, 2018-2030 (USD Million)
Figure 33 PEEP valve Market, 2018-2030 (USD Million)
Figure 34 Others Market, 2018-2030 (USD Million)
Figure 35 Manual Resuscitators Market: Patient Type segment dashboard
Figure 36 Manual Resuscitators Market: Patient Type Movement Share Analysis
Figure 37 Adult Market, 2018-2030 (USD Million)
Figure 38 Pediatric Market, 2018-2030 (USD Million)
Figure 39 Others Market, 2018-2030 (USD Million)
Figure 40 Manual Resuscitators Market: Application segment dashboard
Figure 41 Manual Resuscitators Market: Application Movement Share Analysis
Figure 42 Chronic Obstructive Pulmonary Disease Market, 2018-2030 (USD Million)
Figure 43 Cardiopulmonary Arrest Market, 2018-2030 (USD Million)
Figure 44 Others Market, 2018-2030 (USD Million)
Figure 45 Manual Resuscitators Market: End Use Segment Dashboard
Figure 46 Manual Resuscitators Market: End Use Movement Share Analysis
Figure 47 Hospital Market, 2018-2030 (USD Million)
Figure 48 Out-of-hospital Market, 2018-2030 (USD Million)
Figure 49 ICUs Market, 2018-2030 (USD Million)
Figure 50 ASC Market, 2018-2030 (USD Million)
Figure 51 Military Market, 2018-2030 (USD Million)
Figure 52 Others (Alternate dispensing site, Closed door) Market, 2018-2030 (USD Million)
Figure 53 Manual Resuscitators Market Revenue, by Region, 2024 & 2030 (USD Million)
Figure 54 Regional Marketplace: Key Takeaways
Figure 55 North America Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 56 Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 57 Canada Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 58 Mexico Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 59 Europe Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 60 UK Manual Resuscitators Market Estimates and Forecasts, 2018-2030,) (USD Million)
Figure 61 Germany Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 62 France Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 63 Italy Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 64 Spain Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 65 Sweden Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 66 Denmark Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 67 Norway Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 68 Asia Pacific Manual Resuscitators Market Estimates and Forecast, 2018-2030 (USD Million)
Figure 69 Japan Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 70 China Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 71 India Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 72 Australia Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 73 Thailand Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 74 Latin America Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 75 Brazil Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 76 Argentina Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 77 MEA Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 78 Saudi Arabia Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 79 UAE Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 80 South Africa Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 81 Kuwait Manual Resuscitators Market Estimates and Forecasts, 2018-2030 (USD Million)
Figure 82 Key Company Categorization
Figure 83 Company Market Positioning
Figure 84 Key Company Market Share Analysis, 2024
Figure 85 Strategic Framework
Companies Mentioned
The major companies profiled in this Manual Resuscitators market report include:- WEINMANN Emergency Medical Technology GmbH + Co. KG
- Laerdal Medical
- Ambu A/S
- Medline Industries, LP
- VYAIRE
- ResMed
- HUM GmbH
- CareFusion (Acquired by BD in 2014)
- Medtronic Plc
- Teleflex Inc.
- Hopkins Medical Products (marketlab)
- PerSys Medical (Safeguard)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 100 |
| Published | February 2025 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 569.07 Million |
| Forecasted Market Value ( USD | $ 799.25 Million |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |