Speak directly to the analyst to clarify any post sales queries you may have.
A concise orientation to the clinical, regulatory, and operational forces driving safety needle prioritization across acute care and home delivery channels
The safety needle landscape is now defined by a convergence of clinical necessity, regulatory scrutiny, and shifting delivery models that together elevate device selection from a procurement exercise to a strategic safety imperative. Needlestick injuries remain a persistent occupational hazard, prompting healthcare systems to revisit device policies and to prioritize engineered safety features that reduce exposure risk and improve user experience. Simultaneously, the expansion of home-based care and self-administration practices has broadened the settings in which these devices must perform reliably and intuitively.
Clinical teams, supply chain leaders, and product developers are navigating a more complex decision matrix that balances user safety, ease of use, sterilization standards, and environmental impact. Technological differentiation such as passive activation mechanisms and ergonomic designs has become integral to product value propositions, while regulatory approvals and procurement contracts increasingly favor demonstrable safety outcomes. As a result, manufacturers are pressured to integrate human factors engineering, quality assurance, and robust post-market surveillance into development timelines.
Looking forward, stakeholders who align product design with end-user workflows and regulatory expectations will be better positioned to influence purchasing decisions and to support safer care delivery across acute, outpatient, and home environments. In this context, the safety needle category is transitioning from commodity status to a specialty segment where clinical evidence, supply reliability, and total cost of ownership inform long-term adoption.
How tightening regulatory standards, supply chain realignment, and user-centric design innovation are redefining competitive advantage in the safety needle market
The past several years have produced transformative shifts in the safety needle ecosystem, driven by legislative momentum, supply chain realignment, and accelerating user expectations. Regulatory authorities have tightened device evaluation and post-market reporting requirements, resulting in a higher bar for both clinical evidence and manufacturing rigor. This has propelled a shift from active safety mechanisms that rely on clinician engagement toward passive designs that automatically mitigate exposure, reflecting a broad industry preference for fail-safe solutions.
Concurrently, supply chain strategies have evolved in response to geopolitical pressures and procurement resilience initiatives. Manufacturers and buyers alike are emphasizing shorter, more diversified supplier networks and enhanced inventory management practices. The trend toward localized manufacturing and strategic nearshoring has gained traction, particularly for critical components such as stainless steel needles and high-grade polymers. These shifts are accompanied by sustainability objectives that push firms to explore recyclable materials and reduced packaging waste while preserving sterility and performance.
Digital health trends have also altered market dynamics. Remote care and telehealth expansion have increased demand for devices suited to lay-user administration, prompting design changes focused on intuitive handling and safety lockouts. Taken together, these shifts create a landscape where regulatory compliance, supply resilience, user-centric design, and environmental considerations define competitive advantage and long-term adoption.
Understanding the strategic supply chain, procurement, and operational responses to the cumulative tariff interventions affecting medical device sourcing in 2025
Tariff actions implemented in 2025 have introduced an added layer of complexity to procurement and manufacturing strategies within the safety needle sector. Import duties on certain medical components and finished devices have influenced cost structures and led purchasing organizations to reassess total landed cost and supplier relationships. Procurement teams are responding by conducting more rigorous supplier risk assessments and expanding qualification criteria to include geographic diversification, dual sourcing, and contingency capacity.
As tariffs alter the relative economics of cross-border sourcing, manufacturers have accelerated conversations about production redistribution and contract manufacturing partnerships in jurisdictions with favorable trade arrangements. These adjustments are not purely transactional; they often require redesigning logistical flows, revisiting regulatory filings tied to country-of-origin declarations, and updating quality management system documentation to reflect new production footprints. In addition, tariff-driven cost pressures encourage closer collaboration between suppliers and buyers to identify opportunities for component standardization and material optimization that can soften the impact on downstream purchasing.
Ultimately, the cumulative effect of tariffs is to raise the strategic importance of supply chain agility and procurement sophistication. Stakeholders who proactively model tariff scenarios, embed flexibility into sourcing strategies, and invest in near-term operational changes will mitigate disruption and preserve predictable access to critical safety devices.
Insights into how product type, material composition, clinical application, end-user environment, and distribution channels collectively shape design and procurement strategies
Differentiation across product type is increasingly decisive: active safety mechanisms that require user engagement continue to serve environments with trained clinical staff, while passive systems that automatically deploy safety features gain favor where human error risk and staffing variability are higher. This dichotomy shapes product development priorities and procurement criteria, with active devices prized for cost efficiency in controlled settings and passive devices valued for error-proofing in mixed-use contexts.
Material choice further influences performance and sustainability narratives. Metal components remain the default for needle integrity and sharpness retention, whereas plastic elements enable integrated safety housings and ergonomic shaping. The balance between metal and plastic usage therefore informs manufacturing techniques and end-of-life considerations, as well as regulatory documentation for biocompatibility and sterilization validation.
Application segments exhibit distinct usage patterns and clinical expectations. Blood collection devices demand precision and compatibility with laboratory workflows, insulin delivery systems prioritize user-friendly interfaces and adherence support for chronic use, and vaccination programs require high-throughput designs optimized for mass immunization campaigns. These application-driven requirements cascade into procurement specifications and clinician training programs.
End-user environments drive both design and commercial strategies. Clinics and hospitals typically emphasize device lifecycle costs, compatibility with clinical workflows, and institutional purchasing agreements, while home care settings elevate ease of self-administration, packaging, and patient education. Distribution channels mirror these preferences: hospital pharmacies focus on institutional bundles and contract fulfillment, retail pharmacies cater to over-the-counter accessibility and consumer trust, and online channels-encompassing company websites and third-party platforms-enable direct-to-consumer access with opportunities for subscription models and value-added digital services. The interaction of these segmentation dimensions shapes product roadmaps, commercialization strategies, and post-market support investments.
Regional contrasts that dictate differentiated commercialization, manufacturing footprint, and regulatory engagement approaches across the Americas, EMEA, and Asia-Pacific
Regional dynamics exert meaningful influence on adoption patterns and strategic priorities within the safety needle category. In the Americas, procurement ecosystems are characterized by sophisticated hospital networks, concentrated purchasing power, and an emphasis on occupational safety standards that drive institutional demand for advanced safety mechanisms. Home care and chronic disease management trends in the region also support a growing need for devices that combine ease of use with robust safety features.
Across Europe, Middle East & Africa, regional heterogeneity defines divergent priorities. Western European markets often emphasize regulatory conformity, clinical evidence, and tender-based procurement processes that favor devices with demonstrated cost-effectiveness and safety profiles. The Middle East exhibits investment-driven growth in healthcare infrastructure and an appetite for high-quality devices, while parts of Africa reflect constrained procurement budgets and an emphasis on supply reliability and cold-chain logistics for vaccination campaigns.
The Asia-Pacific region functions as both a major manufacturing base and a dynamic market for adoption. Manufacturing concentration in select economies supports global supply, but regional demand is rising across emerging markets as immunization initiatives expand and primary care infrastructure grows. Local regulatory frameworks and varying reimbursement models create a patchwork of entry strategies, leading manufacturers to balance centralized production with localized distribution partnerships and regional regulatory engagement.
These regional distinctions underscore the need for tailored market approaches that account for procurement mechanisms, regulatory landscapes, infrastructure variability, and end-user expectations.
How innovation, strategic partnerships, manufacturing resilience, and value-added services determine which firms capture long-term procurement preference and clinical trust
Competitive advantage in the safety needle sector is increasingly tied to a combination of technological differentiation, manufacturing excellence, and go-to-market agility. Leading firms invest in passive safety technologies, human factors research, and robust quality systems to meet both regulatory expectations and clinician preferences. At the same time, new entrants are challenging incumbents with niche innovations, lower-cost manufacturing models, or digital-enabled services that enhance training and adherence.
Strategic partnerships and targeted acquisitions continue to serve as accelerants for capability-building. Collaborations between device manufacturers and specialty contract manufacturers enable capacity scaling and localized production, while alliances with clinical networks support evidence generation and adoption. Companies that establish clear intellectual property positions, coupled with transparent regulatory pathways, are better positioned to secure institutional contracts and to defend against commoditization.
Service elements increasingly complement product portfolios. Training programs, digital onboarding tools, and bundled consumable supply agreements strengthen customer relationships and create recurring revenue opportunities. Firms that align commercial teams with clinical educators and procurement specialists can translate product advantages into defensible purchasing preferences, particularly in tender-driven or value-based procurement environments.
In summary, company-level strategies that integrate product innovation, supply reliability, clinical validation, and commercial support are most likely to sustain advantage as purchasing criteria evolve.
Practical strategic actions for manufacturers and purchasers to strengthen product value, supply resilience, regulatory alignment, and user adoption across channels
Industry leaders should prioritize a sequence of coordinated actions to secure market position and respond to evolving clinical and regulatory demands. First, accelerate development and commercialization of passive safety designs that minimize reliance on operator steps while documenting real-world safety outcomes through targeted clinical studies. This evidence base will support procurement negotiations and facilitate inclusion in institutional formularies.
Second, build supply chain resilience by diversifying suppliers, qualifying secondary production sites, and exploring nearshoring where feasible. These measures reduce exposure to trade policy shifts and logistics disruptions while enabling more responsive inventory management. Third, engage proactively with regulatory authorities and procurement bodies to align product specifications with evolving standards and to streamline market entry processes across jurisdictions.
Fourth, invest in end-user training and digital support tools that enhance correct usage in both clinical and home settings, thereby reducing misuse and promoting adherence. Fifth, incorporate sustainability objectives into product design and packaging decisions, balancing material choices with sterilization and performance requirements to meet buyer expectations without compromising safety. Finally, tailor commercialization strategies by region and channel, leveraging hospital pharmacy relationships for institutional sales, retail partnerships for consumer accessibility, and online platforms for direct-to-user distribution with subscription options and patient support services.
Taken together, these actions create a cohesive strategy that addresses safety priorities, mitigates supply risks, and enhances commercial traction across diverse customer segments.
A rigorous mixed-methods research framework combining expert interviews, regulatory synthesis, supplier analysis, and scenario planning to ensure robust insights and transparency
The research approach underpinning this analysis combined qualitative and quantitative techniques to produce a robust, triangulated view of the safety needle landscape. Primary research included structured interviews with clinicians, procurement leaders, supply chain specialists, and regulatory experts to surface real-world decision criteria and to capture nuanced adoption barriers. Supplier engagements and manufacturer-level briefings provided visibility into innovation pipelines, capacity constraints, and material sourcing considerations.
Secondary research synthesized regulatory guidance documents, clinical studies, and public procurement policies to contextualize primary findings and to validate thematic trends. Comparative analysis across product types, materials, applications, end users, and distribution channels enabled segmentation-driven insights that reflect operational and clinical realities. Data quality was reinforced through cross-validation of interview inputs against documented product specifications and regulatory filings.
Analytical methods included scenario planning for supply chain and tariff impacts, human factors assessment frameworks for usability evaluation, and comparative value analyses to understand procurement trade-offs. The study also acknowledged limitations related to variability in regional procurement practices and the evolving nature of trade policy; therefore, findings emphasize strategic themes and operational implications rather than prescriptive forecasts. Rigorous documentation of sources and methodological assumptions supports transparency and allows stakeholders to replicate or extend the analysis for specific decision needs.
A strategic synthesis of safety, supply chain, and regulatory priorities that clarifies pathways for durable adoption, procurement efficiency, and clinical impact
The safety needle sector stands at an inflection point where clinical safety imperatives, regulatory pressures, and supply chain realities intersect to reshape product design, procurement, and commercialization strategies. Passive safety mechanisms and user-centered designs are increasingly favored as institutions and home-care providers seek reliable, error-resistant devices. At the same time, tariffs and geopolitical shifts heighten the need for diversified manufacturing footprints and more sophisticated procurement practices.
Regional variation underscores the importance of tailored strategies: sophisticated buyers in developed markets demand robust clinical evidence and lifecycle cost analyses, whereas emerging regions prioritize supply continuity and affordability. Companies that combine technological differentiation with resilient supply networks, regulatory engagement, and targeted commercial support will better meet stakeholder expectations and sustain adoption.
For purchasers, the imperative is to move beyond unit price comparisons and to evaluate total cost, clinical outcomes, and supplier reliability. For manufacturers, the priority is to align product roadmaps with clinical workflows, to invest in evidence generation, and to create flexible manufacturing and distribution strategies that absorb policy and market shocks. Stakeholders who embrace these principles will be well-positioned to improve patient and clinician safety while navigating a complex, rapidly evolving landscape.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Safety Needles Market
Companies Mentioned
The key companies profiled in this Safety Needles market report include:- AdvaCare Pharma
- Argon Medical Devices, Inc.
- Artsana Group
- B. Braun SE
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Cartel Healthcare Pvt. Ltd.
- Eli Lilly and Company
- Gerresheimer AG
- Hindustan Syringes and Medical Devices Ltd.
- Medtronic PLC
- Merit Medical Systems, Inc.
- Nanchang Kindly(KDL) Medical Technology Co., Ltd.
- Nipro Corporation
- Novo Nordisk A/S
- Retractable Technologies, Inc.
- Retrago Technologies
- Smiths Medical, Inc.
- Socorex Isba SA
- Sol-Millennium Medical Inc.
- Terumo Europe NV
- UltiMed, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
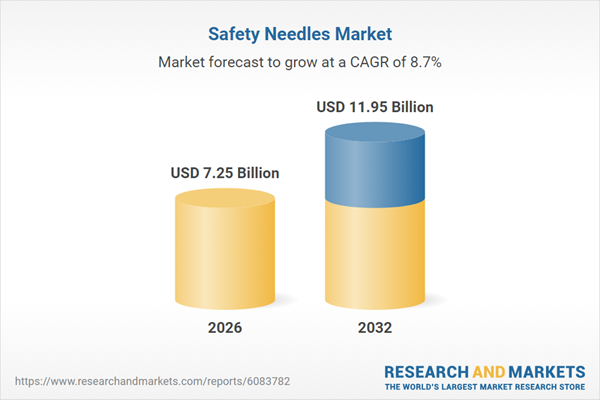
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 7.25 Billion |
| Forecasted Market Value ( USD | $ 11.95 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |