Speak directly to the analyst to clarify any post sales queries you may have.
The sexually transmitted disease diagnostics market is rapidly evolving, driven by technological advancements, shifting regulatory landscapes, and an urgent need for accessible and accurate diagnostic solutions. Senior decision-makers must navigate a sector in transformation, where informed strategies will define successful market positioning.
Market Snapshot: Sexually Transmitted Disease Diagnostics Market Overview
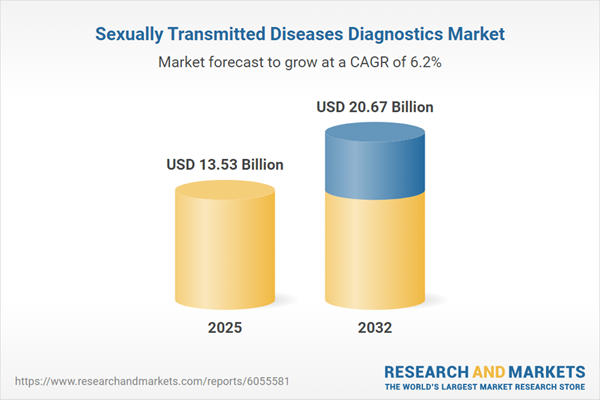
The sexually transmitted disease diagnostics market expanded from USD 12.78 billion in 2024 to USD 13.53 billion in 2025 and is forecast to reach USD 20.67 billion by 2032 at a CAGR of 6.18%. Growth is fueled by rising global infection rates, the introduction of new diagnostic technologies, and increased prioritization of early and equitable access in healthcare systems. Health organizations are rapidly embracing innovative, rapid, and sensitive diagnostic tests and decentralized care models. These strategies are improving public health outcomes, optimizing workflows, and enhancing patient access across diverse markets.
Scope & Segmentation
- Technology: Encompasses traditional bacterial culture and microscopy, advanced nucleic acid amplification including PCR and isothermal methods, and novel rapid diagnostics such as lateral flow assays, microfluidics, and serological tests.
- Disease Coverage: Addresses a broad spectrum of infections, such as Chlamydia, Gonorrhea, Herpes, HIV, Human Papillomavirus, and Syphilis, to align with evolving global health priorities.
- End User Profiles: Focuses on clinics, hospitals, diagnostic laboratories, and research institutions across public and private sectors, enabling scalable and targeted deployment.
- Distribution Channel: Extends reach via direct sales, distribution networks, and online platforms, supporting access in both developed and emerging market environments.
- Test Type: Facilitates both screening and confirmatory testing for laboratory and point-of-care applications, enhancing flexibility to clinical and operational needs.
- Sample Type: Integrates use of blood, swab, and urine specimens to support compatibility with multiple diagnostic workflows and care settings.
- Regional Markets: Reflects varied adoption trends in the Americas, Europe, Middle East, Africa, and Asia-Pacific, influenced by regulatory, reimbursement, and infrastructure dynamics.
- Company Coverage: Features analysis of key industry players such as F. Hoffmann-La Roche Ltd, Abbott Laboratories, Danaher Corporation, Hologic Inc., bioMérieux SA, QIAGEN N.V., Thermo Fisher Scientific Inc., Siemens Healthineers AG, Becton, Dickinson and Company, and Cepheid Inc.
Key Takeaways: Strategic Insights for Decision-Makers
- Advances in diagnostic platforms are enabling timely test results in both point-of-care and centralized laboratory settings, aligning with evolving clinical requirements and public health needs.
- The integration of digital health solutions and unified data systems is facilitating more streamlined diagnosis, personalized treatment routes, and improved patient management across geographies.
- Strengthened resilience in manufacturing and sourcing strategies is mitigating operational risks and managing costs, particularly in response to changing international trade dynamics.
- Awareness of regional nuances such as reimbursement structures and local regulations is key to effective go-to-market strategies and adoption planning.
- Collaboration among technology firms, academia, and healthcare providers is expediting research output, improving product development, and driving the rollout of next-generation diagnostics.
- The availability of both screening and confirmatory test formats, compatible with diverse sample types, positions organizations to address a broader spectrum of patient and clinical needs.
Tariff Impact: Navigating Supply Chain Challenges
Recent tariffs in the United States affecting diagnostic devices and reagents have driven organizations to adjust procurement strategies and pursue more localized manufacturing. This ongoing shift requires continual review of cost structures and sourcing approaches, influencing the competitive landscape and pricing trends within the sexually transmitted disease diagnostics market.
Methodology & Data Sources
The market analysis employs interviews with established experts and rigorous evaluation of regulatory and scientific sources. Quantitative insights are strengthened through triangulation, referencing leading government, industry, and international health organization data for accuracy and depth.
Why This Report Matters
- Delivers comparative analysis and highlights emerging areas for expansion within the sexually transmitted disease diagnostics ecosystem.
- Empowers senior leaders with the intelligence needed to guide investment, forge strategic partnerships, and address supply chain and regulatory fluctuations.
- Provides timely insights into technology adoption and evolving demand patterns across established and high-growth regions.
Conclusion
The sexually transmitted disease diagnostics sector is navigating ongoing innovation, expanding regional presence, and increased collaboration. Consistent focus on these pivotal areas empowers organizations to broaden diagnostic access and drive efficient outcomes.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Sexually Transmitted Diseases Diagnostics market report include:- F. Hoffmann-La Roche Ltd
- Abbott Laboratories
- Danaher Corporation
- Hologic, Inc.
- bioMérieux SA
- QIAGEN N.V.
- Thermo Fisher Scientific Inc.
- Siemens Healthineers AG
- Becton, Dickinson and Company
- Cepheid Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 13.53 Billion |
| Forecasted Market Value ( USD | $ 20.67 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |