Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative overview framing the clinical, technological, and commercial context shaping the adoption and strategic importance of stereotactic surgery devices
Stereotactic surgery devices have become an indispensable component of modern neurosurgical, ENT, and spine practice, enabling clinicians to navigate complex anatomy with precision and confidence. Recent advances in imaging, tracking technologies, and robotic assistance have elevated the procedural capabilities available to surgeons, reducing operative time, improving accuracy, and expanding the range of minimally invasive interventions. Today’s stakeholders confront a landscape where clinical outcomes, regulatory pathways, procurement cycles, and reimbursement dynamics intersect, making timely, evidence-based intelligence essential for strategic decision-making.As hospitals and outpatient facilities refine care pathways and prioritize value-based outcomes, the role of navigation systems, robotic platforms, and complementary accessories has expanded beyond niche use cases to core procedural support. This shift places a premium on interoperability, ease of use, and demonstrable clinical benefit, while vendors must balance innovation with practical considerations such as sterilization workflows, capital procurement, and staff training. Consequently, commercial strategies that emphasize clinical partnerships, real-world evidence generation, and modular product architectures are gaining traction.
This introduction establishes the foundational context for stakeholders assessing market opportunities, competitive dynamics, and operational challenges. The narrative that follows synthesizes technology trends, policy impacts, segmentation insights, and regional nuances to inform investment, product development, and commercialization strategies across the stereotactic surgery ecosystem.
A synthesis of technological convergence, clinical evidence demands, and regulatory evolution driving a next-generation transformation in stereotactic device adoption
The stereotactic device landscape is undergoing transformative shifts driven by converging technological, clinical, and commercial forces that are redefining what is possible in precision surgery. First, tracking and guidance technologies are migrating from single-modality solutions toward integrated ecosystems that combine electromagnetic and optical tracking with advanced software for image fusion, enabling more robust intraoperative decision support. This trend reduces procedure variability and supports expanded indications in delicate anatomical regions.Second, robotics and automation are maturing from assistive tools to workflow-centric platforms that emphasize surgeon control, haptic feedback, and seamless OR integration. The emphasis on modular robotic architectures and software-driven upgrades allows providers to scale capabilities without replacing entire capital assets. As a result, procurement decisions are increasingly influenced by upgrade paths, third-party compatibility, and lifecycle cost management.
Third, the convergence of real-world clinical evidence, outcome-driven contracting, and post-market surveillance is elevating the importance of demonstrable value. Payers and healthcare systems are asking for clear evidence linking device utilization to patient outcomes and cost efficiencies, prompting vendors to invest in clinical trials, registry participation, and health economics analyses. Finally, regulatory expectations around cybersecurity, software as a medical device, and data governance are shaping design and commercialization timelines, creating both barriers and differentiation opportunities for compliant, innovative solutions.
How 2025 tariff adjustments reshaped sourcing strategies, procurement models, and product design considerations across the stereotactic device value chain
The tariff environment in the United States during 2025 introduced elevated cost considerations that ripple across procurement, supply chain configuration, and pricing strategies within the stereotactic device sector. Import duties on select components and subassemblies intensified scrutiny of supplier footprints and stimulated reassessment of nearshoring and local manufacturing options. Consequently, procurement teams and manufacturers revisited vendor contracts, total landed cost models, and inventory policies to mitigate exposure to tariff volatility.These tariff-induced pressures accelerated supply chain diversification, with vendors seeking alternative sourcing routes and higher component standardization to preserve margins. To maintain price competitiveness, manufacturers adjusted product architectures to increase the proportion of domestically sourced or tariff-exempt materials where feasible, and invested in quality-controlled subcontractor networks within tariff-friendly jurisdictions. At the provider level, capital acquisition timelines were scrutinized more closely, and cost-benefit analyses incorporated updated procurement scenarios that reflect potential long-term duty implications.
While tariffs created near-term cost headwinds, they also incentivized strategic shifts that can yield long-term resilience: stronger supplier partnerships, investments in regional manufacturing capabilities, and product designs that facilitate serviceability and component interchangeability. For stakeholders planning multi-year product roadmaps or service expansions, integrating tariff sensitivity analysis into commercial and operational planning has become indispensable to safeguarding margin and ensuring uninterrupted clinical adoption.
Segment-driven analysis that interlinks end user characteristics, tracking technologies, product classes, device architectures, and clinical applications to guide commercial prioritization
Insights grounded in segmentation reveal nuanced demand drivers and adoption pathways across end users, technologies, product categories, device types, and clinical applications. When considering end user dynamics, ambulatory surgical centers, hospitals, and specialty clinics exhibit distinct investment horizons and workflow expectations; ambulatory surgical centers are subdivided into hospital-based ASCs and independent ASCs, while hospitals are differentiated into private hospitals, public hospitals, and teaching hospitals, each presenting unique purchasing protocols and clinical priorities. Technology segmentation clarifies that electromagnetic tracking and optical tracking follow separate innovation trajectories; electromagnetic tracking itself bifurcates into high frequency and low frequency approaches, and optical tracking differentiates into infrared-based and laser-based systems, with each subcategory offering trade-offs in line-of-sight dependency, interference susceptibility, and setup complexity.Product segmentation further refines market engagement: accessories, navigation systems, and robotic systems play complementary roles in procedural enablement. Navigation systems are composed of hardware modules and software components that must be validated for interoperability and regulatory compliance, while robotic systems divide into active and passive platforms that vary by autonomy level, control ergonomics, and integration demands. Device type influences clinical workflow and clinician preference with frame-based devices centering on stereotactic frames and frameless devices offering marker-based and markerless strategies, the latter gaining attention for reduced patient preparation time and enhanced imaging compatibility.
Application segmentation anchors adoption in clinical need: brain surgery procedures including deep brain stimulation and tumor resection, ENT procedures such as sinus surgery and tumor surgery, and spine interventions focused on decompression and fusion all impose distinct accuracy, instrument access, and imaging integration requirements. Understanding how these segmentation layers interact is essential for prioritizing product development, designing clinical evidence strategies, and tailoring go-to-market approaches that reflect the purchaser’s operational reality and patient population.
A comparative regional perspective that contrasts adoption enablers, procurement dynamics, and regulatory complexities across the Americas, Europe Middle East & Africa, and Asia-Pacific
Regional insights reveal divergent innovation ecosystems, procurement behavior, and regulatory landscapes that materially influence adoption pathways. The Americas region demonstrates a propensity for rapid clinical uptake where reimbursement frameworks and consolidated healthcare systems favor investments in devices that improve outcomes and reduce length of stay. Providers in this region often demand robust post-market data and favor vendors that can deliver comprehensive service and training packages to support rollout across networked facilities.In Europe, Middle East & Africa, heterogeneity across national health systems shapes procurement cadences and access to capital. Some markets prioritize cost containment and standardized solutions, while others emphasize pioneering clinical programs in academic centers, creating pockets of early adoption. Vendors operating here must navigate varied regulatory frameworks, prioritize local partnerships for distribution and servicing, and design pricing models adaptable to public tender processes and private hospital purchasing.
Asia-Pacific represents a rapidly evolving market characterized by strong investments in hospital infrastructure, growing neurosurgical capacity, and an appetite for technology transfer and local manufacturing. Healthcare systems across the region vary in reimbursement maturity, but many are accelerating adoption of minimally invasive and precision-guidance technologies to meet rising demand for complex procedures. Across all regions, success depends on aligning clinical evidence generation, training programs, and supply chain models with local operational realities to secure durable adoption.
A competitive intelligence perspective highlighting strategic differentiation through clinical partnerships, service networks, and interoperable product architectures
Competitive dynamics center on the interplay between established medtech players, specialized navigation and robotics firms, and emerging technology entrants that challenge incumbents with software-centric offerings. Leading companies differentiate through clinical partnerships, breadth of installed base, and service capability, often leveraging cross-platform compatibility and modular upgrades to reduce switching costs for customers. These firms invest heavily in surgeon education, multicenter registries, and outcome studies to validate clinical benefit and strengthen payer conversations.Smaller, agile entrants focus on niche opportunities such as markerless tracking, compact robotic modules, or specialized instrument suites that address specific procedural bottlenecks. Their advantage lies in rapid iteration, customer responsiveness, and targeted collaborations with key opinion leaders to demonstrate clinical efficacy. Meanwhile, partnerships between imaging vendors, navigation specialists, and robotics providers are becoming more common, enabling integrated solutions that streamline intraoperative workflows. For stakeholders evaluating partners or acquisition targets, attention should be paid to IP strength, regulatory track record, service network scale, and the ability to deliver continuous software updates and cybersecurity assurances.
Ultimately, company strategies that combine strong clinical evidence, scalable service infrastructure, and interoperable product architectures are best positioned to win in an environment where provider expectations extend beyond hardware to encompass lifecycle support and demonstrable clinical value.
A prioritized playbook of practical steps for manufacturers and providers to strengthen evidence, design modular offerings, and fortify supply chain and service capabilities
Industry leaders should prioritize a set of pragmatic actions to convert strategic intent into measurable outcomes. First, invest in clinical evidence programs that target high-impact procedures across representative clinical settings to demonstrate outcomes and economic value; align study designs with payer endpoints and incorporate registry data to accelerate adoption. Second, pursue modular product strategies that enable incremental upgrades and third-party integrations, thereby reducing procurement friction for capital-constrained customers and creating recurring revenue opportunities through software and services.Third, assess and diversify supply chains to mitigate tariff and geopolitical risks, including selective nearshoring for critical components and validation of alternate suppliers to ensure continuity. Fourth, expand training, service, and remote support capabilities to enhance customer retention and accelerate time-to-value; virtual training modules and tele-support can reduce onboarding time while maintaining clinical confidence. Fifth, forge strategic partnerships with imaging vendors, EHR providers, and robotic specialists to deliver interoperable, workflow-centric solutions that match how clinicians operate in the OR.
By sequencing these actions-evidence generation, modular design, supply chain resilience, service expansion, and strategic partnerships-companies can reduce adoption barriers, preserve margin under cost pressures, and create differentiated value propositions that resonate with both clinicians and procurement stakeholders.
A transparent explanation of research sources, stakeholder interviews, and analytical approaches used to derive strategic insights and practical implications for decision-makers
This research synthesizes primary interviews with clinical leaders, procurement specialists, and device engineers, complemented by secondary analysis of peer-reviewed clinical literature, regulatory filings, and publicly available corporate disclosures. Primary inputs were structured to capture perspectives across acute care hospitals, ambulatory surgical centers, and specialty clinics to reflect differences in capital cycle, clinical throughput, and operational constraints. Interviews emphasized real-world workflow, training burdens, and clinical endpoints that influence device selection.Secondary analysis focused on regulatory guidance documents, published clinical trial outcomes, and technological white papers to validate trends in tracking accuracy, robotic control paradigms, and software capabilities. Supply chain and tariff impacts were assessed through trade policy reviews, supplier disclosures, and scenario analysis that models procurement decision levers without extrapolating market sizing. Throughout the methodology, triangulation was used to reconcile any discrepancies between primary feedback and documentary evidence, ensuring conclusions are robust and operationally relevant.
Limitations include the rapidly evolving nature of software and regulatory guidance, which may alter timelines or technical requirements; however, methodological rigor and diverse stakeholder engagement provide a reliable basis for strategic decision-making and scenario planning.
A cohesive conclusion synthesizing clinical imperatives, technological progress, regulatory pressures, and operational levers that determine long-term success in the sector
In conclusion, stereotactic surgery devices inhabit a dynamic intersection of clinical need, technology innovation, and commercial complexity. Advances in tracking systems, robotic assistance, and software integration are expanding clinical possibilities, while regulatory and reimbursement expectations are elevating the importance of demonstrable outcomes and secure software practices. Tariff pressures have accelerated supply chain reconfiguration and underscored the value of flexible product designs and localized manufacturing strategies.For stakeholders across the value chain, the imperative is to couple technological ambition with rigorous clinical validation, resilient operational planning, and customer-centric service models. Product strategies that emphasize modularity and interoperability, combined with robust clinical evidence and scalable service offerings, are most likely to achieve sustainable adoption. Regional nuances in procurement, regulatory frameworks, and clinical capacity require tailored commercialization approaches that reflect local priorities and resource constraints.
Taken together, these insights suggest that companies who invest in evidence, partner effectively, and design for adaptability will secure the greatest long-term advantage in the evolving stereotactic surgery device landscape.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Stereotactic Surgery Devices Market
Companies Mentioned
The key companies profiled in this Stereotactic Surgery Devices market report include:- Accuray Inc.
- Adeor Medical AG
- Brainlab AG
- Computerized Imaging Reference Systems, Inc.
- Elekta AB
- FHC Inc.
- Harvard Bioscience, Inc.
- Hitachi Medical Systems
- IBA
- Inomed Medizintechnik GmbH
- Integra Life Sciences
- Koninklijke Philips N.V.
- Medtronic PLC
- Micromar Ind.
- Modus Medical Devices
- Monteris Medical
- Nexstim PLC
- Nico Corporation
- PMT Corporation
- Raysearch Laboratories
- Renishaw PLC
- Siemens Healthcare Private Limited
- Stayker Corporation
- Zeiss Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
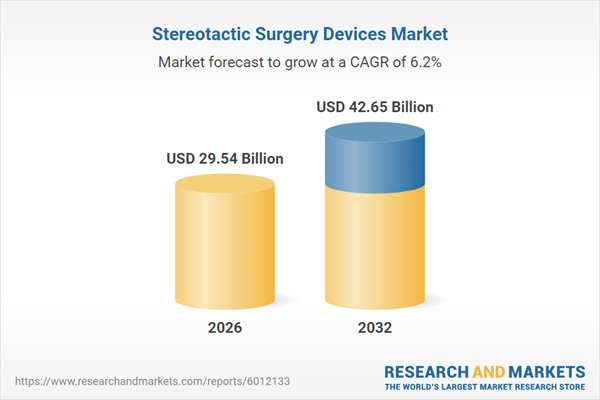
| Estimated Market Value ( USD | $ 29.54 Billion |
| Forecasted Market Value ( USD | $ 42.65 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |