Speak directly to the analyst to clarify any post sales queries you may have.
Concise orientation to how advanced post processing solutions integrate imaging, computation, and clinical workflows to transform acute and longitudinal stroke care
Stroke imaging post processing software sits at the intersection of clinical urgency and computational innovation, enabling faster, more accurate interpretation of complex neuroimaging studies and supporting time-sensitive therapeutic decisions. Advances in algorithmic detection, automated perfusion mapping, and diffusion analysis have transformed how radiologists and stroke teams triage patients, supplementing clinical judgment with quantitative outputs that can be integrated into acute workflows. This introductory overview situates the technology within current care pathways, highlighting how modern software modules interact with CT and MRI data, integrate with hospital information systems, and support multidisciplinary care teams.
Beyond acute decision support, software platforms increasingly support longitudinal care through chronic monitoring, rehabilitation tracking, and risk stratification features. These capabilities reflect an evolving expectation that digital tools provide not only snapshots for immediate treatment but also longitudinal data to inform secondary prevention. As a result, procurement conversations now require attention to interoperability, validation across imaging vendors, and the availability of cloud or on-premise deployment models that align with institutional security and latency requirements.
This introduction frames the subsequent analysis by clarifying core functions, typical deployment architectures, and the clinician-centric outcomes these systems aim to deliver. It also underscores how regulatory scrutiny, payer expectations, and integration complexity shape adoption timelines and vendor selection criteria.
How AI maturation, hybrid deployment trends, and stricter clinical validation standards are collectively transforming expectations for stroke imaging post processing
The landscape for stroke post processing software is being reshaped by several transformative shifts that extend from foundational imaging innovations to systemic changes in healthcare delivery. First, the maturation of artificial intelligence and machine learning has moved beyond proof-of-concept studies to embedded inference engines that accelerate lesion detection, quantify perfusion deficits, and provide automated scoring that clinicians can act upon. These algorithmic advances are complemented by improved image acquisition protocols and vendor-neutral processing pipelines that reduce variability across devices.
Second, the balance between cloud-based services and on-premise deployments continues to evolve. Cloud architectures deliver scalable compute for retrospective analysis and large-scale model retraining, while on-premise solutions address latency, data residency, and integration with proprietary imaging hardware. This duality has prompted hybrid architectures and partnerships between imaging OEMs and software providers to deliver cohesive clinical workflows.
Third, regulatory frameworks and standards for clinical validation are becoming more rigorous, prompting vendors to invest in prospective studies, multicenter validation, and explainability features in algorithm outputs. Concurrently, clinical pathways are shifting toward earlier intervention and expanded indications for thrombectomy and other reperfusion therapies, increasing demand for reliable automated decision support. These shifts collectively create new expectations for accuracy, robustness, and interoperability, pressing vendors and health systems to align product roadmaps with clinical needs and compliance obligations.
Comprehensive assessment of how 2025 United States tariff measures have altered procurement dynamics, supply resilience, and deployment choices for imaging software and hardware ecosystems
The tariff environment instituted by United States policy actions in 2025 introduced new layers of complexity for supply chains, procurement, and vendor economics across medical software and hardware ecosystems. These trade measures affected hardware-dependent modules-such as high-performance servers, specialized GPUs, and integrated workstation components-creating immediate procurement strain where sourcing was previously global and cost-optimized. As a result, software vendors and health systems have confronted elevated acquisition costs for turnkey imaging workstations and accelerated timelines for localizing critical hardware sourcing or qualifying alternative suppliers.
In response, software vendors have pursued multiple mitigation strategies. Some have increased investment in software-only solutions that decouple performance from proprietary hardware, optimizing compute for commonly available accelerators and enabling cloud or virtualized deployments that can shift compute burdens away from tariff-exposed imports. Others have restructured supply chains by diversifying manufacturing partners and establishing regional distribution hubs to reduce exposure to cross-border levies and logistical bottlenecks. Health systems, faced with procurement constraints, have adopted longer evaluation cycles and prioritized vendor flexibility in integration and update pathways.
Regulatory and compliance implications also emerged as organizations sought to maintain validated configurations while changing hardware suppliers. The need to requalify systems for clinical use and ensure that algorithm performance remained consistent across new hardware variants added time and cost to deployment. Overall, the tariffs accelerated strategic decisions around vendor partnerships and architecture choices, encouraging stakeholders to emphasize modularity, vendor-neutral interoperability, and resilient procurement strategies.
Deep dive into modality, deployment, end user, application, and integration segmentation to reveal clinical requirements and procurement implications across solution variants
A nuanced appreciation of segmentation is essential to understand demand drivers, clinical adoption pathways, and product design priorities across stroke post processing software. When viewed through the lens of modality, offerings must address both CT and MRI workflows; within CT, the suite of capabilities spans angiography and perfusion analysis, and perfusion outputs break down into cerebral blood flow, cerebral blood volume, and mean transit time measures, each requiring validated algorithms and consistent mapping to clinical decision pathways. MRI pathways emphasize diffusion weighted imaging with apparent diffusion coefficient quantification alongside perfusion analyses that may leverage dynamic contrast enhanced or dynamic susceptibility contrast techniques, necessitating flexibility in algorithms and robust artifact mitigation.
Delivery mode is another critical axis, where cloud based solutions coexist with on premise deployments; cloud based architectures differentiate between private cloud and public cloud models, while on premise implementations vary from OEM integrated systems to standalone configurations, implicating considerations of latency, data residency, and IT governance. End users encompass ambulatory and specialty centers, diagnostic centers, and hospitals and clinics, with hospital settings further delineated between community hospitals and tertiary referral centers that demand higher integration and advanced analytic features for complex cases.
Application segmentation separates acute stroke assessment from chronic monitoring. Acute pathways focus on urgent decisions such as intravenous thrombolysis and mechanical thrombectomy selection, whereas chronic monitoring targets rehabilitation tracking and risk assessment to guide secondary prevention strategies. Integration options range from integrated workstations to standalone software packages; integrated workstations may be OEM integrated or vendor neutral, while standalone software can be offered as OEM-branded solutions or by third-party developers, influencing procurement, support models, and lifecycle management.
Regional intelligence highlighting how divergent healthcare structures, regulatory regimes, and infrastructure priorities shape deployment and adoption strategies across global markets
Regional dynamics materially influence adoption patterns, regulatory expectations, and partnership approaches for stroke post processing software. In the Americas, clinical networks are increasingly focused on streamlining acute workflows and integrating pre-hospital data, with payers and systems emphasizing demonstrable improvements in care efficiency; this context favors solutions that can be validated in diverse clinical settings and that support rapid interoperability with electronic health records and stroke registries. Across Europe, Middle East & Africa, heterogeneity in regulatory frameworks and infrastructure maturity creates a mosaic of adoption scenarios, from high-acuity tertiary centers prioritizing advanced algorithmic features to resource-constrained settings seeking cost-effective, robust solutions that require minimal local maintenance.
Asia-Pacific presents a broad spectrum of clinical environments where rapid modernization of imaging fleets is occurring in parallel with strong investments in AI-driven diagnostics. Local regulatory bodies are developing frameworks for software-as-medical-device approvals, prompting vendors to engage in region-specific clinical validation and localization efforts. Throughout these regions, reimbursement policies, talent availability for radiology and interventional teams, and national strategies for stroke systems of care shape vendor go-to-market approaches, partnership models, and deployment timelines. Consequently, regional intelligence must guide product configuration choices and commercial positioning to align with distinct clinical and infrastructural realities.
Focused analysis of vendor differentiation, partnership strategies, and service models that determine competitive advantage and adoption pathways in clinical systems
Competitive dynamics among vendors supplying stroke post processing solutions revolve around technological differentiation, clinical validation, and the ability to integrate within complex hospital ecosystems. Established imaging OEMs leverage hardware relationships and installed bases to offer tightly integrated workstations and validated clinical pathways, while software-first vendors compete on algorithmic performance, cloud scale, and rapid update cycles. New entrants and specialized algorithm developers are accelerating innovation in niche areas such as perfusion quantification and automated clot detection, frequently partnering with clinical sites to generate real-world evidence and refine models.
Strategic partnerships between imaging manufacturers, cloud providers, and clinical networks are common, aiming to combine strengths in imaging acquisition, secure data infrastructure, and clinical workflow expertise. Vendors that demonstrate transparent validation, explainable outputs, and robust post‑market surveillance capabilities are better positioned to navigate regulatory scrutiny and hospital procurement processes. Moreover, demand for vendor-neutral solutions is rising among health systems that operate heterogeneous imaging fleets and seek to avoid lock-in, which has prompted a segment of companies to prioritize interoperability standards and open APIs as cornerstones of their commercial propositions.
Finally, service and support models-covering training, implementation, and lifecycle management-are differentiators in procurement decisions, especially for community hospitals and diagnostic centers that may lack in-house informatics capabilities. Companies that offer scalable support and clear evidence of clinical impact generate greater trust and facilitate broader adoption.
Practical and prioritized recommendations for vendors and health systems to accelerate validated adoption while securing supply chain resilience and interoperability
Industry leaders should pursue actions that align product capability with clinical utility while reinforcing operational resilience and regulatory compliance. Prioritize investment in rigorous, multicenter clinical validation and post-market performance monitoring to substantiate claims and support regulatory approvals. Combine explainability features with streamlined clinician interfaces to accelerate trust and reduce cognitive load in acute care settings, recognizing that adoption is as much about workflow fit as it is about analytic accuracy.
Architect products for deployment flexibility by supporting hybrid cloud and on-premise configurations, enabling customers to balance latency, security, and compute needs. Strengthen supply chain resilience through diversification of hardware partners and regional fulfillment capacities to mitigate exposure to trade disruptions. Invest in vendor-neutral interoperability and open APIs to appeal to health systems with heterogeneous imaging environments, and design partnership models that align incentives across imaging OEMs, cloud providers, and clinical networks.
Finally, embed comprehensive implementation and training services into commercial offerings, and collaborate with payers and clinical leaders to build the evidence base linking software use to care process improvements. These combined actions will position leaders to meet clinician expectations, conform to regulatory demands, and maintain commercial momentum in a competitive and evolving landscape.
Transparent hybrid research methodology combining expert interviews, technical literature synthesis, and data triangulation to ensure credible and reproducible insights
The research draws on a hybrid methodology combining primary qualitative engagements with domain experts and secondary technical synthesis of regulatory guidance, clinical literature, and product documentation. Primary research included structured interviews with radiologists, stroke neurologists, health system IT leaders, and product managers to capture clinical priorities, operational constraints, and deployment experiences. These conversations informed hypothesis generation and prioritized areas for deeper technical review.
Secondary research involved systematic review of peer-reviewed clinical studies, standards guidance on imaging and software-as-a-medical-device, and vendor whitepapers and technical specifications to map capabilities against clinical needs. Data triangulation was used to reconcile clinical opinions, technical claims, and regulatory context, ensuring that insights reflect practice realities rather than promotional materials. Segmentation frameworks were developed iteratively to reflect modality, delivery mode, end user, application, and integration, and regional analyses incorporated policy and infrastructure variables to explain adoption patterns.
Quality assurance procedures included cross-validation of key findings with multiple expert sources, dedicated review of algorithm validation approaches, and documentation of assumptions and limitations. This methodology supports transparent, reproducible conclusions and highlights areas where additional prospective research or real-world evidence would further strengthen confidence.
Synthesis of strategic imperatives and clinical priorities that together determine which post processing solutions will achieve meaningful adoption and sustained clinical impact
In conclusion, stroke post processing software is transitioning from adjunct diagnostic tools to integral components of stroke systems of care, driven by advances in algorithmic capability, greater emphasis on workflow integration, and evolving expectations around clinical validation. Adoption choices are being shaped by modality-specific requirements for CT and MRI processing, flexible deployment preferences spanning cloud and on-premise environments, and the operational realities of diverse end users from ambulatory centers to tertiary hospitals. Regional differences in regulation and infrastructure further influence product configuration and go-to-market tactics.
Vendors and health systems that adopt pragmatic strategies-focusing on rigorous validation, vendor-neutral interoperability, resilient supply chains, and clinician-centered design-will be better positioned to translate technological capabilities into measurable improvements in patient pathways. The interaction of regulatory rigor, payer considerations, and clinical demand necessitates a careful, evidence-driven approach to deployment and commercialization. Ultimately, the most successful solutions will be those that demonstrably reduce time to treatment, integrate seamlessly into existing workflows, and support longitudinal care coordination across acute and chronic stroke pathways.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Stroke Post Processing Software Market
Companies Mentioned
The key companies profiled in this Stroke Post Processing Software market report include:- Agfa-Gevaert N.V.
- Aidoc Ltd.
- Arterys, Inc.
- Blackford Analysis Limited
- Brainomix Limited
- Canon Medical Systems Corporation
- Circle Cardiovascular Imaging Inc.
- Combinostics Oy
- GE HealthCare Technologies, Inc.
- Imbio, LLC
- INFINITT Healthcare Co., Ltd.
- Intrasense SA
- iSchemaView, Inc. (RapidAI)
- Koninklijke Philips N.V.
- Medtronic plc
- MeVis Medical Solutions AG
- MIM Software, Inc.
- Mirada Medical Limited
- NeuroLogica Corporation
- Nihon Kohden Corporation
- Olea Medical Imaging Technologies S.L.
- Siemens Healthineers AG
- TeraRecon, Inc.
- Viz.ai, Inc.
- Zebra Medical Vision Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
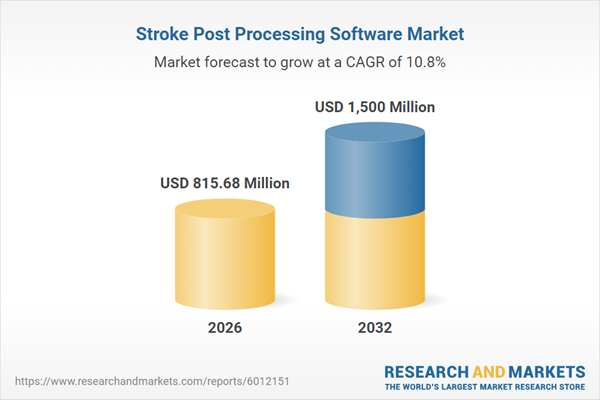
| Estimated Market Value ( USD | $ 815.68 Million |
| Forecasted Market Value ( USD | $ 1500 Million |
| Compound Annual Growth Rate | 10.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |