Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive introduction to support catheter market dynamics, clinical drivers, innovation pathways, regulatory landscape, and care delivery shifts
The support catheter landscape sits at the intersection of procedural innovation and materials engineering, shaped by evolving clinical needs and advances in interventional techniques. Clinicians increasingly rely on these devices to provide access, deliver therapies, and enable precision within complex vascular anatomies, while manufacturers pursue refinements in catheter trackability, torque response, and tip design to support growing procedural diversity. Regulatory frameworks continue to emphasize patient safety and robust clinical evidence, prompting iterative device improvements and expanded labeling across therapeutic indications.As procedural volumes shift toward minimally invasive approaches, the role of support catheters has expanded beyond simple access to become integral to the success of complex interventions. Concurrently, improvements in imaging modalities and adjunctive devices influence catheter design priorities, creating tighter integration between device performance and procedural workflows. Supply chain considerations, including component sourcing and sterilization logistics, now weigh heavily in product planning and commercialization strategies. Consequently, stakeholders must balance clinical performance, manufacturing scalability, and reimbursement realities to ensure devices meet both clinician expectations and health system constraints.
Moving forward, the interplay between clinical evidence generation and practical usability will determine which innovations gain rapid adoption. Manufacturers that align development with procedural needs and streamlined logistics will foster stronger clinician trust and enhance downstream adoption. Therefore, an introductory view of this market emphasizes not only the technical specifications of support catheters but also the procedural, regulatory, and commercial contexts that drive product selection in contemporary practice.
Transformative shifts reshaping the support catheter landscape driven by material science, imaging, invasive therapies and interdisciplinary collaboration
Recent years have produced transformative shifts that are redefining how support catheters are developed, evaluated, and used in clinical practice. Advances in material science and surface engineering have enabled catheters to combine flexibility with column strength, supporting access to tortuous anatomies without sacrificing pushability. Simultaneously, improvements in imaging, including high-resolution intraprocedural visualization, have pushed designers to optimize catheter profiles and radiopacity so devices perform predictably under modern imaging conditions.In parallel, therapy diversification across cardiology, neurovascular, oncology, and peripheral vascular domains has expanded the range of procedural scenarios in which support catheters must perform reliably. This trend is accelerating demand for application-specific designs and has encouraged manufacturers to adopt modular development strategies. Additionally, procedural innovation has been accompanied by workflow optimization efforts within hospitals and ambulatory settings, prompting device makers to focus on ease of use, single-operator compatibility, and sterilization efficiencies.
The commercial environment is also changing: supply chain resilience and nearshoring considerations are influencing component sourcing, while digital channels and service-oriented sales models are altering how products reach end users. Moreover, collaborative relationships between device makers and clinical centers are becoming more strategic, with emphasis on real-world evidence generation and iterative product enhancements. Taken together, these shifts are producing a more dynamic and innovation-driven landscape in which clinical performance, manufacturability, and commercial agility converge.
Assessing the cumulative impact of United States tariffs enacted in 2025 on supply chains, pricing strategies, sourcing, manufacturing and clinical access
Tariff changes implemented in the United States during 2025 have had a cumulative influence on the economics and operational strategies of companies engaged in producing, distributing, and procuring support catheters. At the operational level, increased import duties on components and finished devices introduce higher landed costs, prompting manufacturers to re-evaluate supplier portfolios and consider regional manufacturing alternatives. As a result, organizations are weighing nearshoring and dual-sourcing strategies to mitigate exposure to single-country disruptions and to optimize lead times for high-priority product lines.In commercial terms, these cost pressures translate into tougher negotiations with health systems and distributors as buyers seek to preserve procurement budgets amid constrained capital environments. Consequently, price sensitivity has heightened, and value propositions that emphasize lifecycle cost reductions, device durability, and clinical outcomes gain greater traction. In regulatory and compliance domains, changes to tariff regimes coincide with increased scrutiny on supply chain transparency, encouraging firms to document origin, traceability, and quality controls more rigorously.
Clinicians and health-system procurement teams are adapting by prioritizing supplier relationships that ensure continuity of supply and by rethinking inventory strategies to reduce vulnerability to pricing volatility. For manufacturers, the cumulative effect of tariffs has accelerated investments in supply chain analytics, contract renegotiation, and operational efficiency initiatives. Therefore, while tariffs present near-term headwinds, they also catalyze strategic shifts toward resilience and cost-effective manufacturing practices that can yield long-term competitive advantages.
Key segmentation insights revealing clinical application priorities, material preferences, end user dynamics and distribution channel implications for strategy
Understanding segmentation is essential to identify where clinical needs intersect with commercial opportunity and manufacturing priorities. Based on Type, the market is studied across Diagnostic, Guiding, and Interventional, each category reflecting different performance requirements: diagnostic catheters emphasize atraumatic access and imaging compatibility, guiding catheters prioritize support and stability for device delivery, and interventional catheters balance therapeutic payload compatibility with distal access performance. Based on Application, the market is studied across Cardiology, Neurovascular, Oncology, and Peripheral Vascular. Within Cardiology there are differentiated demands between Coronary and Structural Heart procedures, where coronary interventions require low-profile trackability and structural heart work demands robust support for larger devices. Within Neurovascular the market is further studied across Aneurysm Coiling and Stroke Thrombectomy, two applications that impose extreme navigability and precision requirements. Peripheral Vascular use cases are studied across Lower Extremity and Upper Extremity, where vessel size, anatomical tortuosity, and access site influence design trade-offs. Based on Material, the market is studied across Polyurethane, Ptfe, and Silicone, materials that offer distinct balances of flexibility, lubricity, and biocompatibility and that inform manufacturing and sterilization decisions. Based on End User, the market is studied across Ambulatory Surgical Centers, Clinics, and Hospitals, each end-user setting presenting unique constraints around procedure complexity, inventory management, and purchasing authority. Finally, based on Distribution Channel, the market is studied across Direct Sales, Distributors, and Online, channels that shape commercial engagement models, post-sale service expectations, and pricing dynamics.Collectively, these segmentation lenses reveal where product innovation will have the greatest clinical and commercial impact. For example, interventional and guiding types that serve neurovascular thrombectomy and structural heart procedures often require advanced materials such as PTFE or proprietary coatings and benefit from direct clinical partnerships to validate performance. Conversely, diagnostic catheters deployed in high-volume coronary suites may prioritize cost-effective materials and broad distributor coverage to meet throughput demands. End-user segmentation further clarifies adoption pathways: hospitals and specialized clinics favor devices with extensive clinical evidence and service support, whereas ambulatory surgical centers emphasize streamlined inventory and user-friendly designs. Distribution insights indicate that while direct sales enable close clinical collaboration for complex products, distributor and online channels can accelerate reach for standardized diagnostic catheters. Therefore, an integrated segmentation perspective helps stakeholders prioritize R&D investments, channel strategies, and clinical evidence generation to match device attributes with end-user realities.
Regional perspectives highlighting demand drivers, regulatory environments, manufacturing hubs, reimbursement trends, and clinical adoption across geographies
Regional dynamics materially influence demand patterns, regulatory engagement, and commercialization strategies across the support catheter landscape. The Americas feature robust hospital systems, a strong culture of procedural innovation, and concentrated centers of clinical excellence that drive uptake of advanced interventional devices. Reimbursement mechanisms and established clinical pathways support rapid adoption of new catheter technologies when backed by convincing clinical performance and training programs. Consequently, companies often pilot novel designs in leading centers of excellence within the Americas to establish clinical momentum before broader rollout.Europe, Middle East & Africa present a more heterogeneous regulatory and reimbursement environment, which requires nuanced market entry strategies. Fragmented procurement processes and variable reimbursement conditions call for localized evidence generation and targeted engagement with national regulatory bodies. At the same time, several European markets maintain high procedural volumes and increasingly emphasize cost-effectiveness and long-term outcomes, steering device selection toward solutions that balance initial price with demonstrated clinical value. In the Middle East and Africa, growth corridors are emerging where investments in interventional capability are accelerating demand for devices that are easy to adopt and maintain in resource-constrained settings.
In the Asia-Pacific region, demographic trends and rising prevalence of cardiovascular and neurovascular diseases are expanding the addressable clinical base, while manufacturing ecosystems provide opportunities for strategic partnerships and contract production. Regulatory harmonization efforts in certain markets are reducing time-to-market for validated devices, though local testing and clinical trials remain important for market acceptance. Additionally, commercial channels differ across the region, with some markets favoring distributor relationships and others showing increasing openness to direct engagement and digital channels. Taken together, regional considerations shape prioritization of regulatory investments, manufacturing location decisions, and channel strategies to align product availability with clinical demand and procurement realities.
Competitive company-level insights focused on innovation pathways, strategic partnerships, manufacturing scale, regulatory positioning, and market differentiation
Company behavior within the support catheter domain reflects a blend of sustained R&D, strategic partnerships, and operational scaling to meet clinician expectations and health-system procurement needs. Larger diversified medtech firms leverage broad commercial footprints and integrated clinical networks to support complex product launches and training programs, enabling rapid penetration for catheters that accompany system-level therapies. These organizations typically invest in iterative design improvements, robust clinical studies, and comprehensive service offerings that reduce adoption friction at high-volume sites.Conversely, specialized catheter manufacturers and startups focus on niche performance differentiators-such as proprietary coatings, novel lumen architectures, or unique tip geometries-to address unmet procedural challenges. These smaller players often pursue targeted clinical collaborations and selective market entry strategies to validate their innovations before broad commercialization. Contract manufacturers and component specialists play a critical role by providing scalable production capabilities, precision extrusion, and coating expertise that enable OEMs to compress development timelines and control costs.
Distribution and go-to-market strategies vary. Some companies emphasize direct sales to cultivate clinician relationships and capture feedback for product iteration, while others rely on distributor networks to expand geographic reach efficiently. Digital sales channels and value-added services, including training modules and case support, are increasingly part of competitive differentiation. Across the competitive spectrum, successful companies demonstrate an ability to align product innovation with clinician workflows, secure regulatory clarity, and maintain supply chain resilience, thereby creating a defensible position in a market where clinical performance and operational reliability are preconditions for adoption.
Actionable recommendations that industry leaders can deploy to strengthen resilience, accelerate innovation, optimize supply chains, and expand clinical adoption
Industry leaders can take immediate and pragmatic steps to strengthen market position, support clinician adoption, and mitigate emerging headwinds. First, prioritize clinician-centered design by embedding real-world procedural feedback into iterative product development cycles; this approach improves device usability and shortens the pathway from prototype to routine clinical use. In addition, invest in modular platform strategies that enable rapid adaptation across diagnostic, guiding, and interventional types, thereby maximizing reuse of core components and reducing time-to-market for application-specific variants.Second, fortify supply chain resilience through dual sourcing, nearshoring where feasible, and strategic stock policies for critical components. Implementing advanced supply chain analytics and supplier scorecards will improve visibility into risk exposures and inform contingency planning. Third, align commercial models with end-user preferences by combining direct clinical engagement for complex products with distributor and digital channels for standardized offerings; this hybrid approach balances deep clinical relationships with broad market reach. Fourth, accelerate evidence generation through collaborative clinical studies and registry participation to substantiate clinical value, particularly for applications in cardiology and neurovascular interventions where outcomes data strongly influence procurement decisions.
Finally, pursue partnerships that extend beyond distribution to include co-development, shared manufacturing, and joint training programs. Such collaborations can distribute risk, accelerate innovation, and create bundled value propositions attractive to health systems. By executing on these recommendations, organizations will be better positioned to convert technological advances into sustained clinical adoption and commercial success.
Rigorous research methodology that combines clinical evidence review, expert interviews, regulatory analysis, supply chain mapping, and data triangulation
The research methodology underpinning this analysis integrates primary and secondary approaches, structured to ensure credibility, reproducibility, and practical relevance. Primary research involved interviews with interventional clinicians, procurement leaders, and device engineers to capture contemporary procedural requirements, purchasing behaviors, and performance expectations. These qualitative insights were complemented by targeted discussions with manufacturing and distribution experts to understand production constraints, sterilization protocols, and channel dynamics. Secondary research encompassed a comprehensive review of peer-reviewed clinical studies, regulatory filings, and publicly available technical specifications to validate performance claims and trace innovation trajectories.Data triangulation was used to reconcile findings across sources, and thematic synthesis identified recurring levers that influence adoption, such as material selection, procedural complexity, and reimbursement alignment. Supply chain mapping exercises traced critical component origins and highlighted vulnerabilities that could affect continuity of supply. Where gaps were identified, the methodology incorporated follow-up expert consultations to refine interpretations and ensure that conclusions reflect operational realities. Limitations of the methodology are acknowledged, including variability in regional regulatory regimes and the proprietary nature of certain performance metrics, which can constrain direct comparability across products. Nevertheless, the combined approach yields a robust, evidence-informed view suitable for strategic planning, product development prioritization, and commercial decision-making.
Conclusion synthesizing strategic implications for clinical practice, supply chain resilience, innovation roadmaps, and leadership priorities going forward
This analysis synthesizes the strategic implications of evolving clinical practices, technological advances, and supply chain pressures for stakeholders across the support catheter ecosystem. Clinical demand is diversifying as interventional procedures expand in cardiology, neurovascular, oncology, and peripheral vascular domains, driving a need for tailored catheter designs and materials that reconcile flexibility with support. Technological progress in materials and imaging has raised performance expectations, while tariff and procurement dynamics emphasize the importance of resilient manufacturing and distribution strategies.Consequently, leaders must take a balanced approach that couples innovation with operational rigor. Advancing catheter performance will deliver clinical value only when paired with robust evidence, dependable manufacturing, and accessible commercial pathways. Organizations that invest in collaborative clinical partnerships, flexible manufacturing models, and channel strategies adapted to end-user needs will be positioned to convert product advantages into sustainable adoption. Moreover, proactive supply chain management and clear value propositions aligned with reimbursement priorities will be essential to navigate geopolitical and economic pressures.
In closing, the path to leadership in this domain is defined by the ability to translate technical innovation into measurable clinical benefits while maintaining operational resilience and commercial agility. Stakeholders that execute along these dimensions can expect to influence procedural standards and secure enduring relationships with clinicians and health systems.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Support Catheter Market
Companies Mentioned
- Abbott Laboratories
- Acandis GmbH & Co. KG
- Asahi Intecc Co., Ltd.
- B. Braun Melsungen AG
- Becton, Dickinson and Company
- Biotronik SE & Co. KG
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Cook Medical LLC
- Integer Holdings Corporation
- Johnson & Johnson
- Koninklijke Philips N.V.
- Medtronic plc
- Merit Medical Systems, Inc.
- MicroPort Scientific Corporation
- Stryker Corporation
- Teleflex Incorporated
- Terumo Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
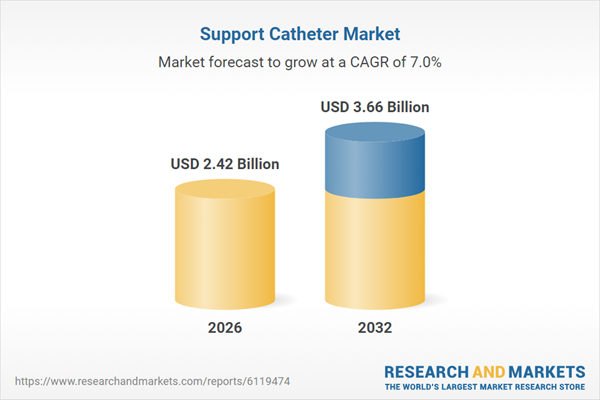
| Estimated Market Value ( USD | $ 2.42 Billion |
| Forecasted Market Value ( USD | $ 3.66 Billion |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |