Speak directly to the analyst to clarify any post sales queries you may have.
A concise clinical and operational framing of acute respiratory distress syndrome that establishes the foundations for strategic device, care pathway, and procurement decisions
Acute respiratory distress syndrome remains one of the most complex and resource-intensive conditions within critical care practice, demanding coordinated clinical protocols, specialized devices, and robust supply chains. While pathophysiology and clinical management continue to evolve, the convergence of advanced extracorporeal technologies, automated patient handling systems, and precision ventilation strategies is reshaping how clinicians approach severe hypoxemic respiratory failure. This report opens with a clear framing of the clinical realities that drive procurement and innovation decisions, highlighting the interplay between device capability, workforce competency, and health system resourcing.Early recognition of deterioration, standardized escalation pathways, and multidisciplinary team coordination are central to improved outcomes, and these operational needs directly influence demand for devices such as extracorporeal membrane oxygenation systems, prone positioning equipment, and a wide array of respiratory consumables. In addition, the growing emphasis on outpatient care models and home-based support for recovery phases is extending the conversation beyond the intensive care unit and into ambulatory and home settings. This requires stakeholders to think holistically about device interoperability, clinician training, and long-term patient monitoring solutions, which in turn informs strategic priorities for manufacturers, providers, and payers alike.
How technological advances, care delivery reorganization, and value-based priorities are fundamentally reshaping the acute respiratory distress syndrome treatment paradigm
The landscape of ARDS care has undergone transformative shifts driven by technological innovation, evidence-based refinements in clinical practice, and evolving health system priorities. Advances in extracorporeal membrane oxygenation have moved beyond specialized centers toward more standardized deployment, while automated prone positioning systems are reducing staff burden and improving safety profiles. Simultaneously, ventilator design has trended toward smarter, more adaptable modes that support lung-protective strategies and individualized settings, and consumables such as airway circuits and filters have become focal points for infection prevention and supply continuity.These technology changes are paralleled by organizational shifts: intensive care units are increasingly integrated with tele-ICU services and remote monitoring platforms, enabling expert oversight across distributed sites. Reimbursement frameworks and procurement policies are emphasizing value-based metrics and outcomes data, incentivizing devices that demonstrably reduce duration of mechanical ventilation or ICU stay. Finally, digital tools that support predictive analytics and clinician decision support are accelerating the move toward personalized ventilation and timely escalation to advanced therapies, creating new opportunities for manufacturers and health systems to collaborate on outcome-driven solutions.
An evaluation of how 2025 tariff policies have reshaped supply chains, procurement dynamics, and manufacturing strategies across the acute respiratory distress syndrome ecosystem
The implementation of United States tariffs in 2025 has introduced a complex set of pressures across the ARDS device ecosystem that extend from component sourcing to hospital procurement strategies. Imported subassemblies and specialized consumables have become more expensive to procure, prompting manufacturers to reassess global supply chains and consider nearshoring or regional manufacturing hubs to mitigate cost volatility. For providers, procurement teams face increased price sensitivity at a time when demand for high-acuity devices remains acute, which has encouraged longer evaluation periods and more rigorous total-cost-of-ownership analyses.In response, some manufacturers are accelerating partnerships with domestic contract manufacturers to secure capacity and reduce exposure to tariff cycles. The tariffs have also amplified interest in modular device architectures and reusable or refashioned components that can be sourced locally. Third-party distributors and e-commerce channels are adjusting commercial terms and inventory strategies to balance margin and availability, while hospitals are strengthening contract negotiations by leveraging group purchasing organizations and longer-term agreements. Clinically, there is renewed emphasis on ensuring that equipment choices are resilient to supply-chain shocks, with an increased appetite for clinically validated alternatives and strategies that support device interoperability and multi-center standardization.
How an integrated segmentation lens across product, therapy mode, care setting, patient demographics, disease drivers, and distribution pathways clarifies strategic priorities
A nuanced segmentation framework reveals distinct commercial and clinical dynamics across product classes, modes of therapy, end users, patient populations, severity strata, etiologies, and distribution channels. Product distinctions between extracorporeal systems, prone positioning solutions, respiratory consumables, and ventilators dictate different regulatory pathways, training requirements, and lifecycle service models; for example, extracorporeal systems require specialized support and clinician expertise whether configured as veno-arterial or veno-venous platforms, while prone positioning options vary materially between automated and manual approaches in terms of staff training and safety protocols. Ventilator portfolios span invasive and non-invasive solutions, with invasive devices differentiated between intensive care and transport use cases and non-invasive devices including bi-level and continuous positive airway pressure modalities, each carrying unique operational footprints and consumable dependencies.Treatment-mode segmentation highlights the clinical decision points among ECMO therapy, invasive and non-invasive ventilation, and prone positioning, and how those pathways interact with resource intensity and training needs. End-use segmentation sheds light on where demand originates: ambulatory care centers and their subtypes such as rehabilitation centers and specialty clinics emphasize post-acute recovery solutions, home care settings require simplified interfaces and remote monitoring, and hospitals including community and teaching institutions drive acute adoption and clinical research collaborations. Patient population segmentation across adult, neonatal, and pediatric groups informs device sizing, regulatory considerations, and clinical trial design, while severity stratification from mild to severe guides escalation pathways and the relative role of advanced therapies. Etiologic differentiation between infectious causes-bacterial, fungal, viral-and non-infectious causes such as aspiration and trauma affects clinical guidelines, infection-control priorities, and the selection of consumables. Finally, distribution-channel segmentation encompassing direct sales, e-commerce, and third-party distributors determines market access strategies, service expectations, and commercial margins, creating differentiated go-to-market models for manufacturers depending on their technology complexity and customer support needs.
Regional adoption patterns, regulatory diversity, and manufacturing footprints that define differentiated pathways to market across the global acute respiratory distress syndrome landscape
Regional dynamics continue to shape adoption patterns, regulatory approaches, and commercial strategies across the ARDS landscape. In the Americas, leading innovation hubs and advanced clinical networks drive early adoption of complex technologies alongside a competitive private provider market; reimbursement considerations and hospital purchasing models influence acquisition strategies and the relative emphasis on service and outcomes support. Europe, the Middle East, and Africa present diverse regulatory and financing environments where public systems and national procurement frameworks often prioritize cost-effectiveness and standardization, creating opportunities for solutions that demonstrate durable clinical benefits and long-term savings. In Asia-Pacific, rapid expansion of critical care capacity, a growing manufacturing base, and rising investments in clinical infrastructure have supported faster uptake of both core ventilator technologies and adjunctive devices, while domestic production capabilities influence supplier ecosystems and price dynamics.Cross-regional collaboration on clinical trials, regulatory convergence initiatives, and manufacturing partnerships is increasing, enabling faster dissemination of best practices and shared approaches to training and quality assurance. However, differences in workforce availability, hospital infrastructure, and reimbursement models require tailored market entry strategies and localization of service offerings to ensure clinical adoption and long-term sustainability in each region.
Competitive and collaborative dynamics among global medtech leaders, specialized innovators, and service-focused entrants that are redefining product strategies and go-to-market models
The competitive landscape is characterized by a mix of integrated multinational medical device companies, specialized niche innovators, contract manufacturers, and agile startups focused on automation, monitoring, and digital platforms. Large device manufacturers maintain portfolios that span advanced extracorporeal platforms through to comprehensive ventilator families, leveraging global service networks and regulatory experience to support complex installations and long-term clinical partnerships. Niche players and startups are contributing breakthrough ideas in areas such as automated patient positioning, disposable consumable improvements, and AI-driven ventilation management, often pairing clinical pilots with rapid iteration cycles to refine usability and workflows.Strategic behaviors among these players include pursuing clinical evidence generation through investigator-initiated studies, forming alliances with academic centers to validate outcomes, and establishing manufacturing partnerships to shore up supply continuity. Mergers, acquisitions, and licensing deals continue to be tools to expand capabilities or accelerate entry into adjacent segments such as home care or remote monitoring. Service models, including equipment-as-a-service and bundled outcomes-based contracts, are gaining traction as manufacturers look to align commercial incentives with clinical results and reduce upfront capital barriers for providers.
Practical strategic moves for manufacturers and health systems to strengthen resilience, accelerate adoption, and align commercial models with clinical value in ARDS care
Industry leaders should prioritize a set of concrete actions to capitalize on clinical needs and market shifts while building resilience against supply-chain and policy pressures. First, invest in modular and interoperable device architectures that reduce dependency on single-source components and enable rapid configuration for different clinical settings. Complement product investments with robust clinician training programs and simulation-based competency initiatives to accelerate safe adoption, especially for high-acuity modalities such as extracorporeal therapies. Second, expand evidence-generation efforts that align with payer and provider outcome metrics, focusing on endpoints that demonstrate reduced ventilation days, lower complication rates, and improved functional recovery.Third, diversify manufacturing and sourcing strategies by exploring regional partnerships and flexible production arrangements to mitigate tariff and logistics volatility. Fourth, develop service-centric commercial models, including managed equipment offerings, training-as-a-service, and remote monitoring subscriptions that create recurring revenue while improving customer retention. Fifth, strengthen interoperability and digital capabilities by embedding data standards and secure connectivity to support tele-ICU integrations and predictive analytics. Lastly, align pricing and reimbursement strategies with value frameworks and engage early with payers and health technology assessment bodies to clarify pathways for coverage and adoption.
A rigorous mixed-methods research framework that integrates literature synthesis, primary interviews, supply-chain mapping, clinical validation, and scenario analysis
The research approach underpinning this analysis combined systematic review of peer-reviewed literature, clinical guidelines, and industry white papers with qualitative primary research comprising structured interviews with intensivists, respiratory therapists, procurement leaders, and device manufacturers. Supply-chain mapping and product pathway analyses were conducted to understand component sourcing risks, manufacturing footprints, and distribution channel behaviors. Clinical adoption patterns were triangulated using hospital case studies and anonymized procurement examples to characterize decision criteria across different end users.Data synthesis employed thematic analysis to identify convergent trends and scenario-based techniques to assess the potential impacts of policy changes, technological maturation, and shifting reimbursement landscapes. Findings were validated through expert panels and follow-up interviews to ensure that conclusions reflect operational realities and current clinical evidence. Throughout the process, methodological rigor was maintained by documenting data sources, interview protocols, and the criteria for inclusion of clinical studies, ensuring transparency and reproducibility of key insights and recommendations.
A concise synthesis of clinical, commercial, and operational imperatives that determine which ARDS innovations will achieve durable adoption and measurable outcomes
The evolving acute respiratory distress syndrome ecosystem demands integrated strategies that bridge clinical efficacy, operational feasibility, and commercial viability. Advances in extracorporeal technologies, automated positioning, and smarter ventilators are expanding therapeutic options, but successful adoption depends on workforce readiness, resilient supply chains, and credible clinical evidence. Tariff-driven cost pressures and regional disparities in regulation and capacity further underscore the need for adaptable manufacturing strategies and localized go-to-market models.Organizations that combine product innovation with outcome-focused service offerings and strong evidence generation will be best positioned to meet provider needs and influence payer decisions. Collaboration across manufacturers, clinical centers, and payers can accelerate the validation of new care pathways and support scalable training programs. Ultimately, balancing technological ambition with pragmatic considerations around deployment, maintenance, and reimbursement will determine which solutions achieve sustained clinical impact and commercial success.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Acute Respiratory Distress Syndrome Market
Companies Mentioned
The key companies profiled in this Acute Respiratory Distress Syndrome market report include:- APEPTICO GmbH
- Armstrong Medical Ltd.
- Athersys, Inc.
- Bayer AG
- Besmed Health Business Corp.
- Drägerwerk AG & Co. KGaA
- EUROSETS S.R.L.
- F. Hoffmann-La Roche Ltd.
- Fisher & Paykel Healthcare Corporation Limited
- GE HealthCare
- General Electric Company
- Getinge AB
- Hamilton Medical AG
- HEALIOS K.K.
- Koninklijke Philips N.V.
- LivaNova PLC
- Medtronic plc
- Mindray Medical International Limited
- Nihon Kohden Corporation
- Novartis AG
- NRx Pharmaceuticals
- Pfizer Inc.
- ResMed Inc.
- Sun Pharmaceutical Industries Limited
- Terumo Medical Corporation
- United Therapeutics Corporation
- WEINMANN Emergency Medical Technology GmbH + Co. KG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
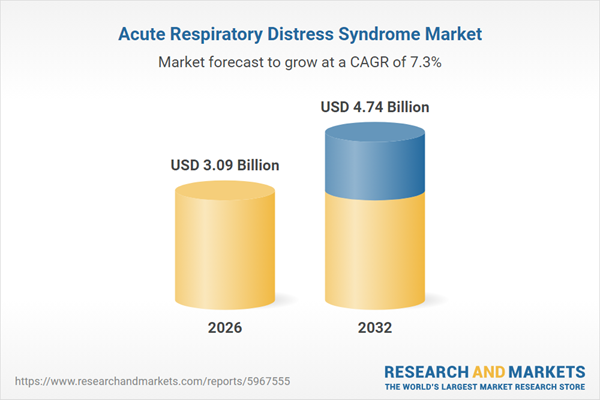
| Estimated Market Value ( USD | $ 3.09 Billion |
| Forecasted Market Value ( USD | $ 4.74 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |