The U.S. liver cancer diagnostics market is experiencing rapid transformation, shaped by rising disease incidence, increasing innovation in early detection, and multiple FDA-backed approvals and designations that are reshaping clinical practice. According to the National Cancer Institute (NCI) and the American Cancer Society, an estimated 42,240 new cases of liver and intrahepatic bile duct cancer are expected to be diagnosed in 2025, with approximately 30,090 deaths projected in the same year. Hepatocellular carcinoma (HCC), the most common form of liver cancer, remains one of the fastest growing and deadliest cancers in the country, with mortality rates continuing to escalate due to late-stage detection and underlying risk factors such as hepatitis B and C, alcoholic liver disease, obesity, and the rising prevalence of non-alcoholic fatty liver disease (NAFLD).
Recent regulatory milestones are reshaping the treatment and diagnostics ecosystem. In July 2025, Sirtex Medical’s SIR-Spheres Y-90 resin microspheres received FDA approval for the treatment of unresectable HCC, making it the only radioembolization therapy approved for both HCC and metastatic colorectal cancer in the United States. Results from the DOORwaY90 clinical trial demonstrated outstanding efficacy, with an overall response rate of 98.5% and a 100% local tumor control rate, positioning SIR-Spheres as a powerful tool in liver-directed therapies.
Complementing therapeutic advances, diagnostics are also breaking new ground. In April 2025, Mursla Bio’s EvoLiver blood test received FDA breakthrough device designation for surveillance of HCC in patients with high-risk cirrhosis, marking the first such test in over five years. Backed by strong data from the MEV01 trial, EvoLiver demonstrated 86% sensitivity and 88% specificity in early-stage detection, significantly outperforming traditional ultrasound and AFP testing. By capturing organ-specific extracellular vesicles and employing multiomics biomarkers, EvoLiver introduces a more convenient, non-invasive, and highly accurate surveillance method with the potential to transform patient adherence and outcomes.
Strategic collaborations further highlight the market’s trajectory. In June 2025, FUJIFILM Medical Systems U.S.A. and Helio Health partnered to advance blood-based assays for early HCC detection, leveraging the HelioLiver Test in combination with Fujifilm’s µTASWako i30 system. Clinical data from the U.S. CLiMB trial showed higher sensitivity and specificity for early-stage HCC detection compared to existing modalities, underscoring the role of AI-driven biomarker panels in broadening surveillance access.
In parallel, FUJIFILM Healthcare Americas is leading the TRACER study, a large-scale, multi-center NIH-funded trial launched in 2024, evaluating the GALAD score, which combines biomarkers AFP, AFP-L3, and DCP with patient demographics to improve early HCC detection in high-risk patients. By enrolling 5,500 participants with cirrhosis or chronic hepatitis B, the study aims to validate biomarker-driven screening models that could reduce the rate of late-stage diagnoses.
Industry leaders are also focusing on biomarker-driven liquid biopsy platforms. In May 2024, Exact Sciences’ Oncoguard Liver liquid biopsy test, published in Clinical Gastroenterology and Hepatology, demonstrated 82% early-stage sensitivity and 88% overall sensitivity with 87% specificity, outperforming current AFP- and ultrasound-based methods. This test represents a significant advancement in HCC surveillance, with the potential to extend early detection to millions of at-risk Americans who remain unscreened.
U.S. Liver Cancer Diagnostics Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. The analyst has segmented the U.S. liver cancer diagnostics market report based on the test type, and end use:Test type Outlook (Revenue, USD Million, 2021-2033)
- Laboratory Tests
- Biomarkers
- Oncofetal and Glycoprotein Antigens
- Enzymes and Isoenzymes
- Growth Factors and Receptors
- Molecular Markers
- Pathological Biomarkers
- Blood Tests
- Imaging
- Endoscopy
- Biopsy
End use Outlook (Revenue, USD Million, 2021-2033)
- Hospitals & Diagnostic Laboratories
- Academic & Research Institutes
- Pharmaceutical & CRO Laboratories
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the industry across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segmental and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listings for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The major companies profiled in this U.S. Liver Cancer Diagnostics market report include:- Abbott Laboratories
- Thermo Fisher Scientific, Inc.
- F. Hoffmann-La Roche Ltd.
- Qiagen N.V.
- Siemens Healthineers
- Becton, Dickinson & Company
- Illumina, Inc.
- Epigenomics AG
- Koninklijke Philips N.V.
- Fujifilm Medical Systems U.S.A., Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | August 2025 |
| Forecast Period | 2024 - 2033 |
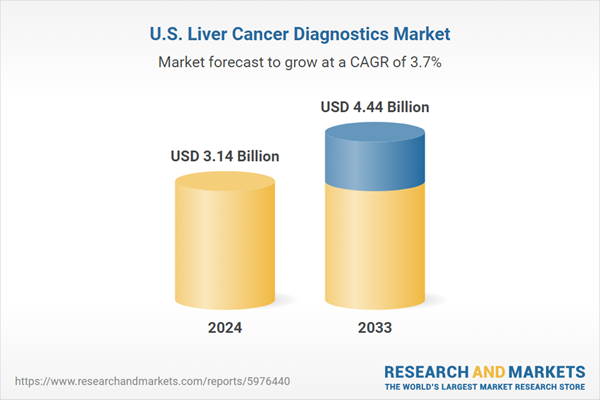
| Estimated Market Value ( USD | $ 3.14 Billion |
| Forecasted Market Value ( USD | $ 4.44 Billion |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | United States |
| No. of Companies Mentioned | 11 |