Global Ursodeoxycholic Acid Market - Key Trends & Drivers Summarized
Why Is Ursodeoxycholic Acid Central to Modern Hepatobiliary Therapies?
Ursodeoxycholic acid (UDCA), a secondary bile acid originally found in bear bile and now synthesized for pharmaceutical use, has become a cornerstone treatment for several chronic liver and gallbladder-related disorders. It plays a pivotal role in managing primary biliary cholangitis (PBC), gallstone dissolution in non-candidates for surgery, and intrahepatic cholestasis of pregnancy (ICP), among other conditions. As the global burden of liver diseases continues to rise - driven by alcohol consumption, metabolic syndrome, hepatitis infections, and autoimmune liver diseases - the need for effective, non-invasive treatments like UDCA is more urgent than ever. Its ability to improve bile flow, reduce bile acid toxicity, and protect hepatocytes makes it a first-line option for many hepatologists. Notably, UDCA's use in pediatrics is expanding, especially in treating neonatal cholestasis and cystic fibrosis-related liver complications. With an aging global population and increasing liver disease screening rates, the clinical relevance of UDCA is only intensifying. Its non-surgical nature, relatively favorable side effect profile, and ability to delay disease progression without immunosuppression make it an indispensable asset in chronic liver care.How Are Innovations in Formulations and Delivery Enhancing Efficacy and Patient Adherence?
Formulation innovation is playing a crucial role in expanding the usability and effectiveness of ursodeoxycholic acid across varied patient demographics. Traditional UDCA tablets and capsules are being reformulated into dispersible, chewable, and liquid forms to cater to pediatric patients and those with dysphagia. Sustained-release and microencapsulated delivery formats are also emerging, designed to offer more stable bile acid levels and better bioavailability over longer durations. In parallel, fixed-dose combinations of UDCA with other bile acid modulators or antioxidants are being developed to enhance therapeutic impact, particularly in patients with advanced liver dysfunction. Additionally, pharmaceutical manufacturers are optimizing excipients to improve gastrointestinal tolerance, one of the main reasons patients discontinue long-term UDCA therapy. These improvements in delivery systems are not only enhancing compliance but also reducing variability in drug absorption, which is critical in chronic, slow-progressing diseases like PBC. Research is also underway to understand the potential synergistic effects of combining UDCA with next-generation agents like FXR agonists or obeticholic acid, potentially ushering in a new era of combination hepatoprotective therapies.Why Are Global Health Trends Creating New Opportunities for UDCA Utilization?
Several macro health trends are aligning to widen the therapeutic and commercial scope of UDCA. First, the surge in non-alcoholic fatty liver disease (NAFLD) and its more severe form, non-alcoholic steatohepatitis (NASH), is prompting off-label interest in bile acid modulators, including UDCA, for their cytoprotective and anti-inflammatory properties. Although not yet formally approved for NASH, UDCA continues to be studied and sometimes prescribed as adjunct therapy in specific cases. Secondly, rising awareness and diagnosis of rare autoimmune liver diseases - facilitated by improved serological testing and patient registries - are expanding the eligible patient pool for UDCA. In obstetrics, greater screening for intrahepatic cholestasis of pregnancy in high-risk groups, including those with twin pregnancies or IVF histories, is increasing UDCA prescriptions as a frontline intervention to reduce perinatal complications. Furthermore, evolving global pharmacovigilance standards are helping clinicians identify and manage adverse drug interactions more effectively, ensuring safer long-term use of UDCA even in polypharmacy scenarios. As public health priorities shift toward early detection and preventive liver care, UDCA's role is being reinforced in both specialist and primary care pathways.What Are the Key Factors Driving Growth in the Ursodeoxycholic Acid Market?
The growth in the ursodeoxycholic acid market is driven by several factors tied to disease prevalence, pharmaceutical innovation, and global healthcare accessibility. Increasing incidence of chronic liver diseases - including PBC, cholestatic liver disorders, and bile acid metabolism abnormalities - is driving demand across both adult and pediatric populations. Advances in drug formulation technologies are enabling wider adoption through patient-friendly delivery formats such as oral liquids and delayed-release tablets, which cater to diverse age groups and comorbidities. The expanding scope of UDCA in managing conditions like ICP and its exploration in NAFLD/NASH management are further propelling demand. Regulatory approvals and updated clinical guidelines in Europe, North America, and Asia-Pacific are legitimizing wider use, especially as newer bile acid therapies remain costlier or less accessible. Additionally, the rise in routine liver function testing and better diagnostic capabilities are allowing earlier initiation of therapy, increasing lifetime treatment value. Pharmaceutical market dynamics, such as the growth of generics and increased API manufacturing in Asia, are lowering treatment costs and supporting market expansion in emerging economies. Collectively, these factors are shaping a robust and upward growth trajectory for the UDCA market.Report Scope
The report analyzes the Ursodeoxycholic Acid market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Dosage Form (Solid Dosage Form, Liquid Dosage Form); Mode of Extraction (Synthetic, Biological); Application (Gastrointestinal Disorders, Liver Disorders, Cystic Fibrosis, Other Applications); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Solid Dosage Form segment, which is expected to reach US$767.7 Million by 2030 with a CAGR of a 11.4%. The Liquid Dosage Form segment is also set to grow at 7.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $155.9 Million in 2024, and China, forecasted to grow at an impressive 14.2% CAGR to reach $212.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Ursodeoxycholic Acid Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Ursodeoxycholic Acid Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Ursodeoxycholic Acid Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Agrata Biotech Ltd., BBT Biotech GmbH, Bharat Serums and Vaccines Ltd., Cadila Pharmaceuticals Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Ursodeoxycholic Acid market report include:
- ABC Farmaceutici SpA
- Adare Pharma Solutions
- ALP Pharm
- Anhui Biochem Pharmaceutical Co., Ltd.
- API Corporation
- Apicore
- Arch Pharmalabs Ltd.
- Asia Pioneer Pharmaceuticals
- Axplora
- Beijing Geyuantianrun Bio-tech Co., Ltd.
- Chengdu Chenlv Biological Technology Co., Ltd.
- Daewoong Pharmaceutical Co., Ltd.
- Dipharma Francis S.r.l.
- Erregierre S.p.A.
- Glenmark Pharmaceuticals Ltd.
- Grindeks
- Guangzhou Tosun Pharmaceutical Co., Ltd.
- Hanways Chempharm Co., Ltd.
- ICE Pharma
- Mankind Pharma Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ABC Farmaceutici SpA
- Adare Pharma Solutions
- ALP Pharm
- Anhui Biochem Pharmaceutical Co., Ltd.
- API Corporation
- Apicore
- Arch Pharmalabs Ltd.
- Asia Pioneer Pharmaceuticals
- Axplora
- Beijing Geyuantianrun Bio-tech Co., Ltd.
- Chengdu Chenlv Biological Technology Co., Ltd.
- Daewoong Pharmaceutical Co., Ltd.
- Dipharma Francis S.r.l.
- Erregierre S.p.A.
- Glenmark Pharmaceuticals Ltd.
- Grindeks
- Guangzhou Tosun Pharmaceutical Co., Ltd.
- Hanways Chempharm Co., Ltd.
- ICE Pharma
- Mankind Pharma Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 468 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
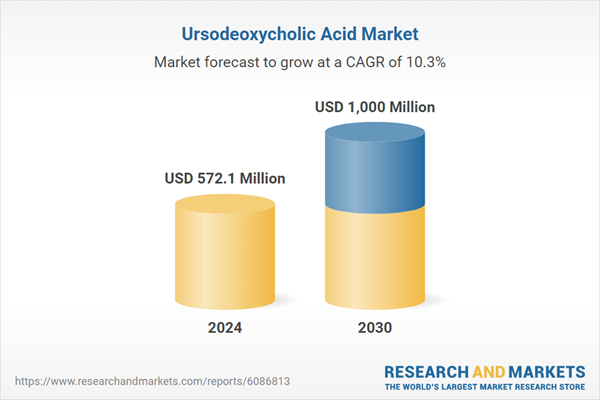
| Estimated Market Value ( USD | $ 572.1 Million |
| Forecasted Market Value ( USD | $ 1000 Million |
| Compound Annual Growth Rate | 10.3% |
| Regions Covered | Global |