Speak directly to the analyst to clarify any post sales queries you may have.
A contemporary framing of clinical complexity, delivery innovation, and patient-driven dynamics shaping the vaginitis therapeutics landscape
The introduction frames the clinical and commercial importance of vaginitis therapeutics at a time of elevated patient awareness, shifting clinical practice, and evolving product innovation. The conditionset spans fungal, bacterial, and inflammatory etiologies, creating a therapeutic landscape where antifungal agents and microbiome-supportive interventions coexist as complementary approaches. Clinicians are balancing empirical treatment with growing diagnostic precision, while patients increasingly seek over-the-counter convenience alongside evidence-based prescription options. These dynamics are reshaping the interplay between pharmaceutical development, retail accessibility, and patient education.
Advancements in formulation science and delivery modalities are altering treatment usability and adherence, with intravaginal, oral, and topical routes each offering distinct advantages in terms of targeted exposure, systemic tolerability, and patient preference. At the same time, demographic shifts and lifecycle considerations are prompting more nuanced segmentation of end users, from pre-menopausal populations to pregnant and post-menopausal cohorts. This segmentation informs clinical trial design, labeling strategies, and commercial messaging. Consequently, stakeholders across manufacturers, distributors, and healthcare providers must align clinical value propositions with pragmatic access pathways to ensure therapeutic uptake and sustained adherence.
This introduction establishes the framework for the subsequent sections by outlining the clinical contexts, delivery innovations, and demographic nuances that collectively shape strategy development for vaginitis therapeutics.
How diagnostic precision, advanced formulations, and microbiome-focused strategies are fundamentally redefining therapeutic approaches and commercial models
The therapeutic landscape for vaginitis is undergoing transformative shifts driven by new clinical insights, formulation breakthroughs, and changing patient expectations. Molecular diagnostics and point-of-care tests are enabling more precise differentiation among candidiasis, bacterial vaginosis, and mixed presentations, which in turn is prompting targeted treatment algorithms and reducing inappropriate empiric therapy. As diagnostic confidence rises, clinicians are more willing to prescribe therapies that match specific pathogens and host conditions, increasing the clinical value of agents with proven specificity.
Concurrently, formulation science is advancing beyond traditional creams and tablets to encompass optimized intravaginal gels, fast-dissolving suppositories, and systemic oral regimens engineered for reduced side effects and improved adherence. These innovations are complemented by a rising interest in adjunctive probiotic strategies that aim to restore or support the vaginal microbiome rather than rely solely on pathogen suppression. Regulatory agencies are also adapting guidance to reflect changing evidence frameworks, encouraging clinical programs that measure patient-reported outcomes and microbiome endpoints.
The commercial implications of these shifts are significant. Market participants that integrate diagnostic partnerships, deliver differentiated formulation value, and articulate evidence-based patient benefits will capture stronger clinical uptake. Transitional strategies that bridge prescription and over-the-counter access while preserving safety and efficacy narratives will be particularly effective in translating scientific advances into broad treatment adoption.
Strategic supply chain responses and commercial adjustments driven by evolving United States tariff policies that affect global sourcing and access strategies
Recent tariff adjustments and trade policy shifts in the United States have introduced new operational considerations for supply chains and procurement strategies across the vaginitis therapeutics value chain. Manufacturers that rely on global sourcing for active pharmaceutical ingredients, excipients, or finished formulations are reassessing supplier portfolios and logistics footprints to mitigate tariff-driven cost pressures. Firms with vertically integrated supply chains or localized manufacturing capacity are positioned to absorb or avoid some of these impacts, while those dependent on cross-border suppliers must evaluate contractual and inventory strategies to sustain supply continuity.
Beyond direct cost implications, tariffs influence strategic decisions about product localization, labeling changes, and distribution routing. Companies are increasingly exploring nearshoring and dual-sourcing to reduce exposure to trade volatility, and they are revisiting pricing architecture across prescription and over-the-counter portfolios to preserve competitive positioning. Payers and distributors may react by adjusting procurement practices and shelf assortment, which could lead manufacturers to strengthen commercial partnerships and renegotiate terms to protect market access.
In response, supply chain resilience and agility have become core strategic priorities. Firms that proactively model tariff scenarios, diversify supplier bases, and invest in regulatory and customs expertise can reduce operational disruption. Importantly, transparent communication with channel partners about cost and timing implications helps preserve trust and ensures patient access continuity during periods of trade policy change.
Deep segmentation analysis revealing how treatment types, delivery routes, product classifications, distribution channels, and demographic cohorts shape therapeutic strategy
Segmentation clarifies which therapeutic approaches and delivery systems are most relevant for different clinical and commercial contexts. Based on treatment type, the landscape is defined by antifungal treatments and probiotic treatments; antifungal options further differentiate into azoles and polyenes, with azoles including clotrimazole, fluconazole, and miconazole, and polyenes encompassing amphotericin B and nystatin. This treatment taxonomy highlights where established small-molecule agents intersect with newer microbiome-supportive approaches, shaping clinical protocols and product development priorities.
Based on route of administration, therapeutic strategies span intravaginal, oral, and topical delivery. Intravaginal applications further manifest as creams, gels, and suppositories, while oral forms take the shape of capsules and tablets, and topical forms include creams, gels, and ointments. These route distinctions are pivotal when assessing adherence drivers, local tolerability, and systemic exposure considerations that inform label claims and clinician preferences.
Product type segmentation separates over-the-counter from prescription offerings, creating distinct regulatory and commercial pathways for market entry and consumer messaging. Distribution channel segmentation covers hospital pharmacies, online pharmacies, and retail pharmacies, each presenting unique access mechanics and relationships with prescribers and consumers. End user demographics segment populations into post-menopausal women, pre-menopausal women, and pregnant women, with post-menopausal groups further divided into ages 46-60 and 60+, and pre-menopausal groups categorized into ages 18-25, 26-35, and 36-45. These demographic layers guide clinical trial inclusion criteria, safety labeling, and marketing positioning to ensure that product benefits are articulated with population-specific relevance.
How regional regulatory frameworks, healthcare infrastructures, and channel dynamics across major global clusters influence commercial approaches and patient access
Regional dynamics materially influence clinical practice, regulatory frameworks, and commercial execution across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, healthcare systems and retail channels often facilitate rapid adoption of over-the-counter solutions alongside clinic-based prescription care, creating opportunities for hybrid go-to-market models that bridge consumer convenience and clinician endorsement. North American regulatory pathways emphasize robust safety and efficacy evidence, while payer considerations shape formulary inclusion and distribution priorities.
In Europe, regulatory harmonization across major markets encourages evidence-standardization and multi-country clinical programs, though national reimbursement systems and cultural attitudes toward self-care create nuanced access patterns. The Middle East & Africa region presents heterogenous regulatory maturity and varying distribution infrastructure, which requires tailored commercial approaches and local partnership strategies to reach both urban and underserved populations. Meanwhile, Asia-Pacific demonstrates a spectrum of innovation receptivity where advanced markets may rapidly adopt novel diagnostics and formulations, while emerging markets rely heavily on cost-effective, accessible therapeutic options. Across all regions, digital channels and online pharmacies are growing as complementary access routes, necessitating integrated omnichannel strategies that respect regional regulatory and cultural differences.
Effective regional playbooks combine global clinical evidence with localized market intelligence, ensuring that product positioning, pricing, and channel strategies align with patient behaviors and healthcare system realities in each geographic cluster.
Competitive behaviors and strategic partnerships among leading developers defining product differentiation, distribution innovation, and patient-centric commercialization
Key companies operating in the vaginitis therapeutics space are pursuing diverse strategies, including lifecycle management of established antifungal agents, investment in probiotic and microbiome-supportive research, and expansion of differentiated delivery technologies. Established pharmaceutical players leverage regulatory expertise and broad distribution networks to defend prescription franchises while introducing reformulations to improve tolerability and convenience. Specialty developers are focusing on targeted formulations and clinical differentiation, often collaborating with diagnostic firms to strengthen product positioning in pathogen-specific treatment pathways.
Across the competitive landscape, partnerships and licensing agreements are common mechanisms to accelerate time-to-market for novel formulations and adjunctive therapies. Manufacturers are also investing in digital therapeutics and telehealth-friendly packaging to align with evolving care delivery models. Distribution strategies increasingly reflect omnichannel thinking, combining traditional hospital and retail pharmacy relationships with direct-to-consumer online channels that emphasize education and privacy.
Companies that prioritize patient-centric design, invest in robust clinical evidence, and build resilient supply chains will create durable competitive moats. Moreover, those that cultivate strong payer relationships and demonstrate clear outcomes in real-world settings will improve access and adherence, reinforcing the commercial viability of both established and emerging therapeutic options.
Actionable strategic priorities for leaders to align diagnostics, formulation innovation, supply chain resilience, and regional go-to-market execution
Industry leaders should adopt a multi-dimensional strategy that aligns clinical differentiation, supply chain resilience, and commercial agility to capitalize on evolving patient and provider needs. First, integrate diagnostic partnerships into product development and launch plans to ensure that clinicians can confidently target therapies to pathogen-specific presentations, thereby enhancing therapeutic precision and reducing inappropriate treatment. Embedding diagnostics into commercial narratives will also facilitate stronger formulary and guideline positioning.
Second, prioritize formulation innovation that improves adherence and tolerability across intravaginal, oral, and topical routes. This includes investing in technologies that enable sustained release, reduced irritation, and patient-friendly administration formats. Concurrently, evaluate opportunities in microbiome-supportive products that complement antifungal agents and address recurrence risk through ecosystem restoration approaches. Such a portfolio strategy balances immediate symptom relief with long-term vaginal health considerations.
Third, strengthen supply chain flexibility through diversified sourcing, nearshoring where feasible, and scenario planning for tariff and trade disruptions. Transparent communication with distribution partners and adaptive pricing strategies will help maintain market access during periods of external volatility. Finally, develop tailored regional go-to-market plans that reflect local regulatory requirements, channel norms, and demographic priorities, while leveraging digital engagement to reach consumers with privacy-sensitive, evidence-based educational content.
A rigorous mixed-methods methodology combining literature synthesis, expert interviews, and competitive landscaping to produce actionable clinical and commercial insights
The research methodology underpinning this analysis integrates a mixed-methods approach combining secondary literature synthesis, targeted expert interviews, and structured competitive landscaping. Secondary sources include peer-reviewed clinical studies, regulatory guidance documents, and publicly available product monographs to ensure clinical assertions and formulation descriptions are grounded in authoritative evidence. Expert interviews with clinicians, formulators, supply chain specialists, and commercial leaders provided qualitative insights into prescribing behavior, patient adherence drivers, and operational constraints.
Competitive landscaping entailed systematic review of product portfolios, formulation attributes, and distribution footprints to map strategic positioning across prescription and over-the-counter categories. Regional regulatory and channel analyses were informed by jurisdictional guidance and payer practice reviews, supplemented by practitioner perspectives to capture real-world access dynamics. The methodological framework emphasized triangulation of evidence to mitigate bias and ensure findings reflect both clinical rigor and commercial practicability.
Limitations include the evolving nature of diagnostics and microbiome science, as well as potential variability in regional implementation timelines. Where appropriate, recommendations are framed to be robust under multiple scenarios and to be adaptable as new clinical data and regulatory clarifications emerge.
Integrated strategic summary emphasizing diagnostic alignment, formulation advantage, and operational resilience as drivers of sustained therapeutic and commercial success
The conclusion synthesizes the strategic imperatives for stakeholders across the vaginitis therapeutics ecosystem. Precision diagnostics, formulation innovation, and patient-centric delivery models are converging to create a more differentiated treatment landscape in which clinical evidence and usability define commercial success. Companies that strategically integrate diagnostics with therapeutics, invest in formulations that enhance adherence, and adopt distribution strategies attuned to regional realities will be best positioned to capture long-term value.
Operational resilience, particularly in supply chain design and tariff scenario planning, is equally critical to sustaining access and preserving competitive continuity. Moreover, segmentation by treatment type, route of administration, product classification, distribution channel, and demographic cohort must inform targeted clinical programs and commercial messaging to ensure relevance and uptake. Execution discipline in clinical development, regulatory engagement, and omnichannel commercialization will determine which organizations convert scientific advances into durable patient impact.
Ultimately, the path forward requires coordinated investment in evidence generation, strategic partnerships, and adaptive commercial models that prioritize therapeutic efficacy, patient experience, and sustainable access across diverse global markets.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Vaginitis Therapeutics Market
Companies Mentioned
The key companies profiled in this Vaginitis Therapeutics market report include:- Bayer Aktiengesellschaft
- Cipla Limited
- Dr. Reddy's Laboratories Ltd.
- Glenmark Pharmaceuticals Ltd.
- Lupin Limited
- Pfizer Inc.
- Sun Pharmaceutical Industries Limited
- Teva Pharmaceutical Industries Ltd.
- The Procter & Gamble Company
- Viatris Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
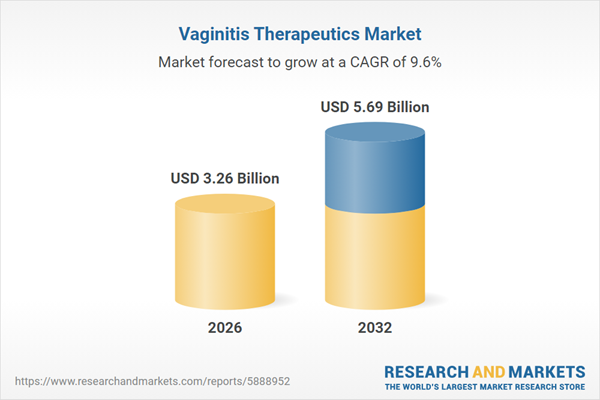
| Estimated Market Value ( USD | $ 3.26 Billion |
| Forecasted Market Value ( USD | $ 5.69 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |